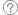
The following is from the University of Western Australia School of Medicine and Pharmacology online module on Formulate a Clinical Question
Cohort studies
These studies take a large population and follow patients who have a specific condition or receive a particular treatment over time and compare them with another group that has not been affected by the condition or treatment being studied. Cohort studies are observational and not as reliable as randomised controlled studies, since the two groups may differ in ways than the variable considered in the study.
Case Control Studies
These are studies in which patients who already have a specific condition are compared with people who don’t. They often rely on medical records and patient recall for data collection. These types of studies are often less reliable than randomised controlled trials and cohort studies because showing a statistical relationship does not mean that one factor caused the other.
Case Series and Case Reports
Consist of collections of reports on the treatment of individual patients or a report on a single patient. Because they are reports of cases and use no control groups with which to compare outcomes, they have no statistical validity and are subject to many biases.
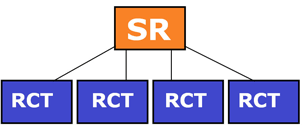
Prospective, blind comparison to a gold standard
These are studies that show the efficacy of a diagnostic test. In these studies, all patients with varying degrees of an illness, or clinical suspicion of an illness, and who are eligible for an investigation or clinical test, are administered both investigations or tests – the test under investigation and the ‘gold standard’ test. The ‘gold standard’ test is investigation or clinical test that has, until now, been shown to be most sensitive/specific for the condition. The investigators reading or interpreting the test results are blinded to the results of the alternative test. Results are usually expressed in terms of ‘sensitivity’ and ‘specificity’, positive and negative predictive value.
Clinical practice guideline
Whilst not a ‘study type’ as such, these documents may attempt to systematically answer a number of questions related to an illness. Many clinical practice guidelines are developed by independent bodies or regulatory organisations. A well-researched clinical practice guideline should use rigorous methodology to ask clinical questions, and systematically review and critically appraise the evidence. A recommendation or guideline should be made and the strength of that recommendation should be given.
http://www.meddent.uwa.edu.au/teaching/acq/lo1acq_fcq/type/pop_types
The following is from the Type of study page of Duke University Medical Center's Introduction to Evidence-Based Practice.
Cross-sectional studies
describe the relationship between diseases and other factors at one point in time in a defined population. Cross sectional studies lack any information on timing of exposure and outcome relationships and include only prevalent cases. They are often used for comparing diagnostic tests.
Retrospective cohort (or historical cohort)
follows the same direction of inquiry as a cohort study. Subjects begin with the presence or absence of an exposure or risk factor and are followed until the outcome of interest is observed. However, this study design uses information that has been collected in the past and kept in files or databases. Patients are identified for exposure or non-exposures and the data is followed forward to an effect or outcome of interest.
Qualitative study
answers a wide variety of questions related to human responses to actual or potential health problems.The purpose of qualitative research is to describe, explore and explain the health-related phenomena being studied.
http://guides.mclibrary.duke.edu/c.php?g=158201&p=1036068
The following is from StatsDirect
Prospective study
A prospective study watches for outcomes, such as the development of a disease, during the study period and relates this to other factors such as suspected risk or protection factor(s). The study usually involves taking a cohort of subjects and watching them over a long period. The outcome of interest should be common; otherwise, the number of outcomes observed will be too small to be statistically meaningful (indistinguishable from those that may have arisen by chance). All efforts should be made to avoid sources of bias such as the loss of individuals to follow up during the study. Prospective studies usually have fewer potential sources of bias and confounding than retrospective studies.
https://www.statsdirect.com/help/basics/prospective.htm

 Watch these videos
Watch these videos Show play log
Show play log
 Show play log
Show play log