Special Circumstances (Optional)
Introduction
This section refers to the 2010 AHA Guidelines for CPR and ECC that addresses management of cardiopulmonary arrest in situations that require special interventions beyond those provided during basic life support (BLS) and advanced cardiovascular life support (ACLS). I tried to outline these special interventions in a simple to understand format with the horizontal and vertical integration of the processes which are involved in those unusual situations.
The scientific material and diagrams are based on the information published by "Circulation" Journal of the AHA:
Cardiac Arrest in Special Situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Terry L. Vanden Hoek, Laurie J. Morrison, Michael Shuster, Michael Donnino, Elizabeth Sinz, Eric J. Lavonas, Farida M. Jeejeebhoy and Andrea Gabrielli. Circulation. 2010;122: S829-S861.
Cardiac Arrest In Pregnancy
The frequency of cardiac arrest in pregnancy is rising with an estimated frequency of about 1:30 000 maternities. Despite pregnant women being younger with fewer morbidities compared to the traditional cardiac arrest patients, the survival rates are poor, about 7%.
During resuscitation of a pregnant woman, health care providers have 2 potential problems:
- the mother
- the fetus.
The best chance of fetal survival is the maternal survival. As for the cardiopulmonary arrest in pregnant patients, rescuers must provide appropriate resuscitation based on the physiological changes during pregnancy.
Prevention is better than cure
The following interventions are the standard of care for treating the critically ill pregnant patient (Class I, LOE C):
- Place the patient in the left-lateral position to relieve compression of the inferior vena cava by the gravid uterus, which can produce severe hypotension that may precipitate arrest in the critically ill patient.
- Give high concentration of oxygen.
- Establish intravenous (IV) access above the diaphragm.
- Assess for hypotension; maternal hypotension that warrants therapy has been defined as a systolic blood pressure 100 mm Hg or 80% of baseline. Maternal hypotension can result in reduced placental perfusion and may lead to reduced cardiac output and eventually cardiac arrest. Preload can adequately be increased by fluids.
- Consider reversible causes of critical illness and treat conditions that may contribute to clinical deterioration as early as possible.
BLS / ACLS Modifications
Patient Positioning
In pregnancy, the patient position has been considered as an important strategy to improve the quality of CPR and resultant cardiac output. The patient has to have a large enough uterus to actually compress the great vessels. About 20 weeks is usually a reasonable guideline, but it is even easier to make a personal judgement.
It has been suggested that manual left uterine displacement, which is done with the patient supine, is as good as or even more efficient than left-lateral tilt in relieving aortocaval compression during CPR.
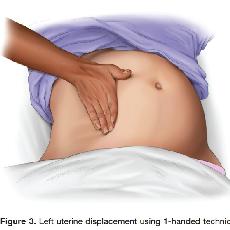
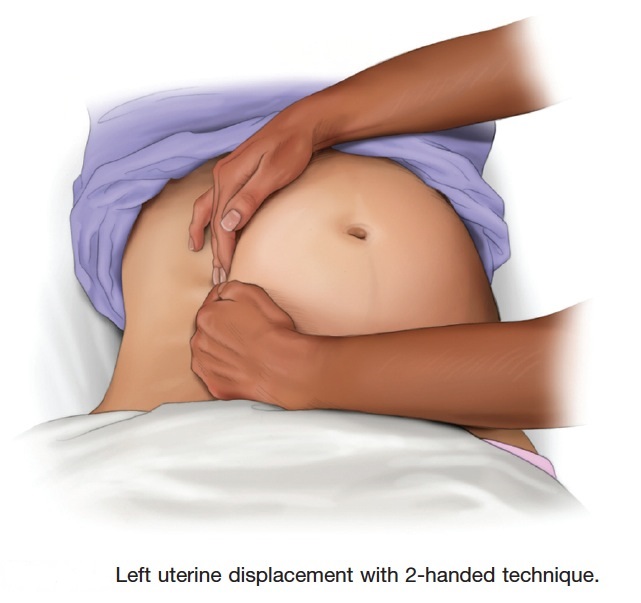
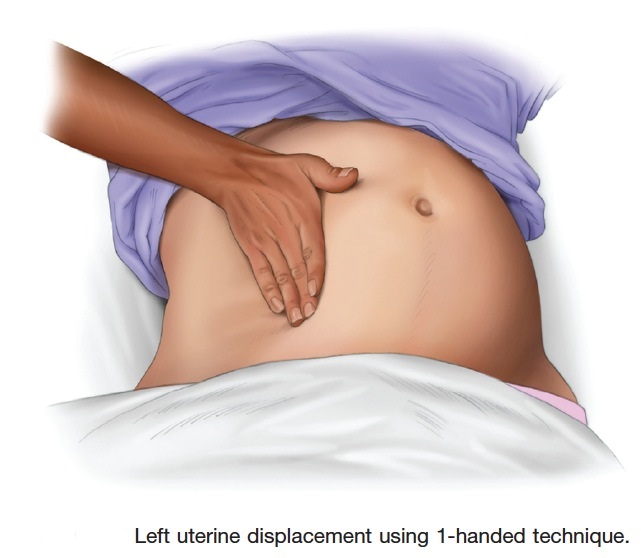
Therefore, to relieve aortocaval compression during chest compressions and optimize the quality of CPR, it is reasonable to perform manual left uterine displacement in the supine position first (Class IIa, LOE C). Left uterine displacement can be performed from either the patient’s left side with the 2-handed technique (Figure 2) or the patient’s right side with the 1-handed technique (Figure 3), depending on the positioning of the resuscitation team.
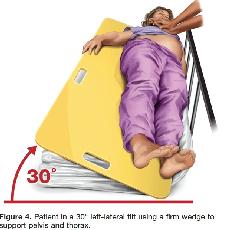
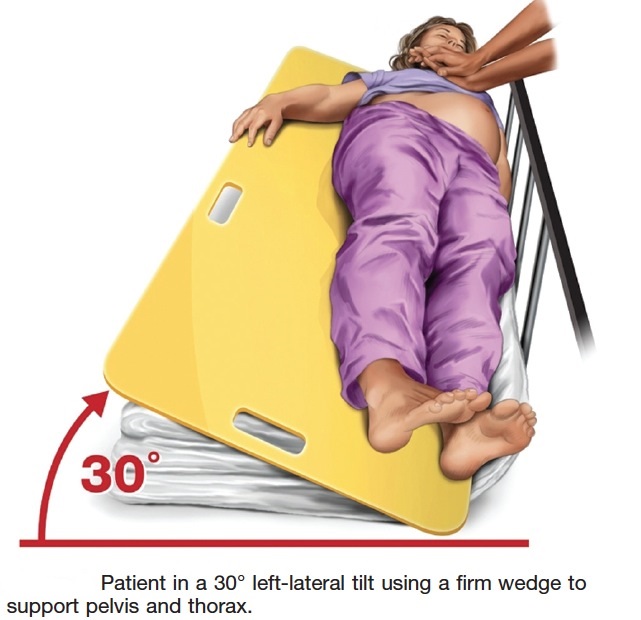
If this technique is unsuccessful, and an appropriate wedge is readily available, then providers may consider placing the patient in a left-lateral tilt of 27° to 30° using a firm wedge to support both the pelvis and thorax (Figure 4) (Class IIb, LOE C).
If chest compressions remain inadequate after lateral uterine displacement or left-lateral tilt, the immediate emergency cesarean section should be considered.
Airway
Airway management is more difficult during pregnancy. This is mainly due to altered airway anatomy, thus increases the risks of aspiration and rapid desaturation.
Pregnancy results in changes in airway mucosa, including edema, friability, hypersecretion, and hyperemia. This results in a smaller upper airway in the third trimester of pregnancy.
Therefore, optimal use of bag-mask ventilation and suctioning is crucial, while preparing for advanced airway placement. During apnea desaturation in pregnant patients is significantly faster than in nonpregnant counterparts. Bag-mask ventilation with 100% oxygen before intubation is especially important in pregnancy (Class IIa, LOE B).
Please note that failed intubation in obstetric anesthesia is a major cause of maternal morbidity and mortality. Endotracheal intubation or supraglottic airway placement should be performed only by experienced providers if circumstances permit.
Breathing
Pregnant patients can develop hypoxemia rapidly because of decreased functional residual capacity and increased oxygen demand. Ventilation volumes may need to be reduced because of the elevated mother’s diaphragm.
Compressions / Circulation
Chest compressions should be performed slightly higher on the sternum (approximately between upper and lower halves of the sternum) than normally recommended (between upper two thirds and lower third) to cater for the elevation of the diaphragm and abdominal contents caused by the gravid uterus.
Changes in Pharmacokinetics
There is an increase in the rate of glomerular filtration and volume of plasma during normal pregnancy. However, there is no solid evidence that current medications and doses for adult patients should be altered during management of cardiac arrest in pregnancy.
Defibrillation
Defibrillation should be performed at the recommended ACLS defibrillation doses (Class I, LOE C). Although there is no evidence of maternal or fetal complications with defibrillation, there are case reports that describe potential harm to the fetus when an accidental electric shock (lightning, electric circuit) is delivered directly to the mother. The greatest predictor of risk for the adverse fetal outcome is if the current travels through the uterus because amniotic fluid most likely transmits current in a manner similar to that transmitted via other body fluids, which could increase the risk of fetal death or burns.
Although there is a small risk of inducing fetal arrhythmias, cardioversion and defibrillation on the external chest are considered safe at all stages of pregnancy. Some experts have raised concern that electric arcing may occur if fetal monitors are attached during defibrillation of a pregnant woman, but there is no evidence to support this.
Overall it is reasonable to assume that if the shock is delivered to the mother’s thorax, there is very low to no risk of electric arcing to fetal monitors. If internal or external fetal monitors are attached during cardiac arrest in a pregnant woman, it is reasonable to remove them (Class IIb, LOE C).
Treatment of Reversible Causes during Cardiac arrest
Magnesium Sulfate Toxicity
Patients with magnesium toxicity present with cardiac effects ranging from:
- ECG interval changes (prolonged PR, QRS and QT intervals) at magnesium levels of 2.5–5 mmol/L
- AV nodal conduction block, bradycardia, hypotension and cardiac arrest at levels of 6–10 mmol/L.
- Neurological effects ranging from loss of tendon reflexes, sedation, severe muscular weakness, and respiratory depression are seen at levels of 4–5 mmol/L.
- gastrointestinal symptoms (nausea and vomiting),
- skin changes (flushing)
- electrolyte/ fluid abnormalities (hypophosphatemia, hyperosmolar dehydration).
Patients with renal failure and metabolic derangements can develop toxicity after relatively lower magnesium doses. During resuscitation empirical calcium administration may be lifesaving in these cases.
Pulmonary Embolism (PE)
Successful use of fibrinolytic in pregnant women has been reported for massive, life-threatening PE and ischemic stroke. Pregnant women in cardiac arrest with suspected PE should be treated in accordance with the ACLS guidelines (see later).
Amniotic Fluid Embolism
Clinicians have reported the successful use of cardiopulmonary bypass for pregnant women with a life-threatening amniotic fluid embolism during labour and delivery. The use of perimortem cesarean section has resulted in maternal and neonatal survival.
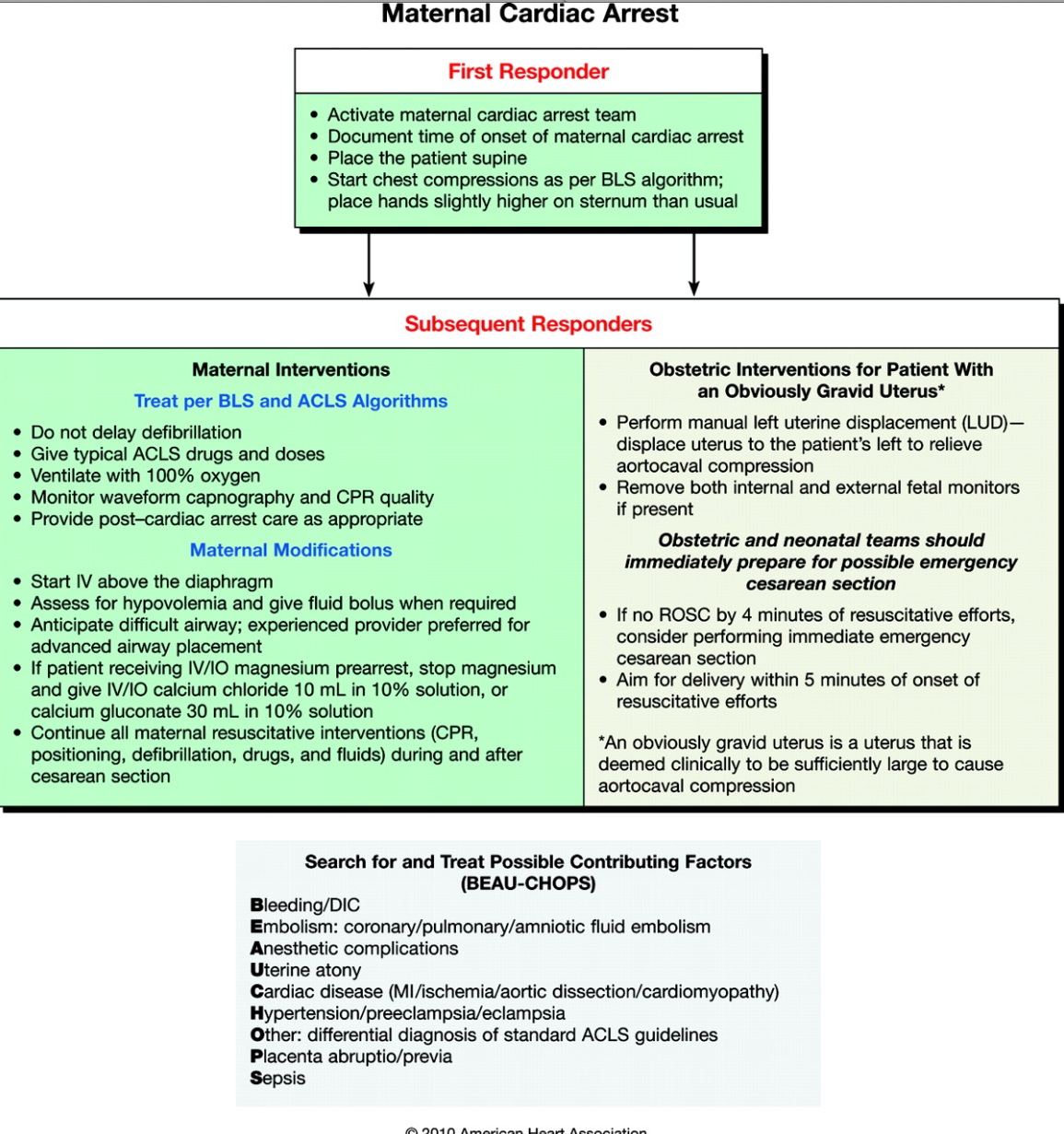
Maternal Cardiac Arrest Not Immediately Reversed by BLS / ACLS
Cardiac arrest resuscitation team leaders should activate the protocol for an emergency cesarean delivery as soon as a cardiac arrest is identified in a pregnant woman with an obviously gravid uterus. By the time the obstetricians are ready to deliver the baby, standard ACLS should be underway and immediately reversible causes of cardiac arrest should be ruled out.
When the gravid uterus is large enough to cause maternal hemodynamic changes due to aortocaval compression, an emergency cesarean section should be considered, regardless of fetal viability.
Aortocaval compression can occur for singleton pregnancies at 20 weeks of gestational age at which the fundal height is approximately at the level of the umbilicus.
Fundal height may also be skewed by other factors such as:
- abdominal distention
increased body mass index
multiple gestation pregnancies
intrauterine growth retardation
For the above reasons, it has been suggested that if the fundus extends above the level of the umbilicus, aortocaval compression can occur, and emergency cesarean section should be performed regardless of gestational age.
Not every pregnant woman in cardiac arrest is a candidate for an emergency cesarean section; the decision depends on whether or not the gravid uterus is thought to interfere with maternal hemodynamics.
Why Perform an Emergency Cesarean Section in Cardiac Arrest?
Several case reports of an emergency cesarean section in maternal cardiac arrest indicate a return of spontaneous circulation or improvement in maternal hemodynamic status only after the uterus has been emptied.
No cases of worsened maternal status after cesarean section were reported. The critical point to remember is that both mother and infant may die if the provider cannot restore blood flow to the mother’s heart as soon as possible.
It has been suggested that a 5-minute window is appropriate for providers to determine if a cardiac arrest can be reversed by BLS and ACLS is not, after which a C-section should be performed while CPR is in progress. However, the rescue team is not required to wait those 5 minutes before initiating emergency hysterotomy, and there are circumstances that support an earlier start.
Survival of the mother has been reported with a perimortem cesarean section performed up to 15 minutes after the onset of maternal cardiac arrest. If emergency cesarean section cannot be performed by the 5-minute mark, it may be advisable to prepare to evacuate the uterus while the resuscitation continues. (Class IIb, LOE C).
Team planning should be done in collaboration with the obstetric, neonatal, emergency, anesthesiology, intensive care, and cardiac arrest services (Class I, LOE C).
Maternal cardiac arrest algorithm
![]()
Perimortem Cesarean Section in the Emergency Department [view/annotate inline]
Hypothermia
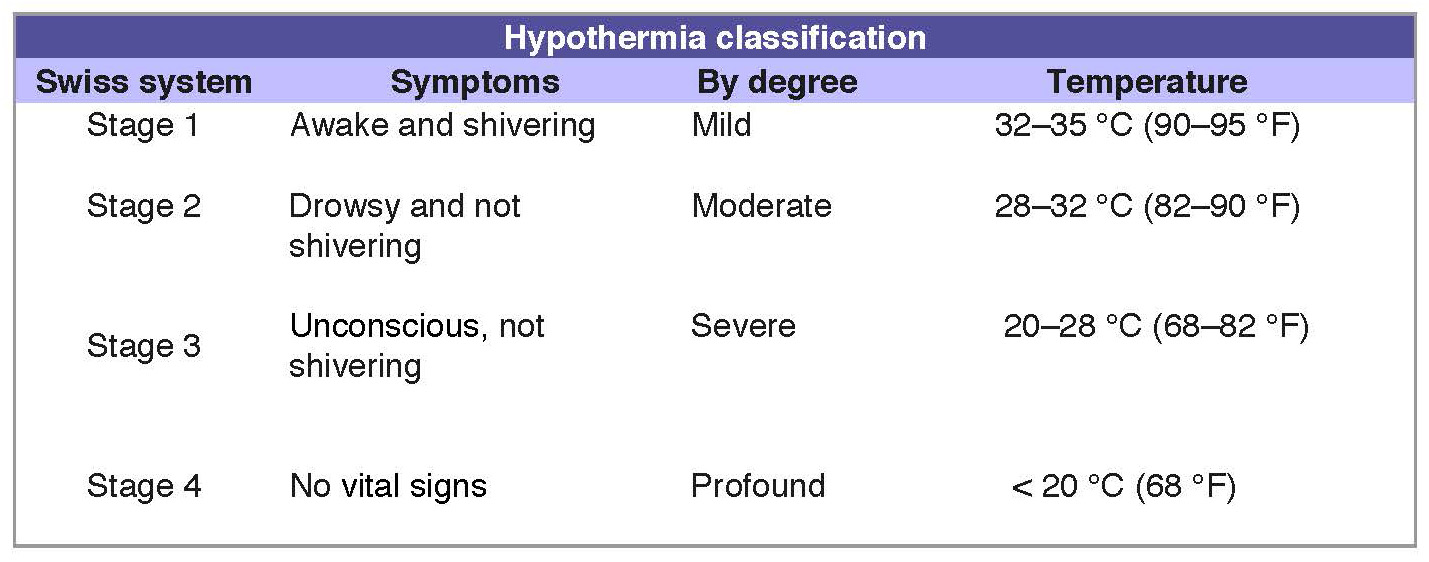
Unintentional or accidental hypothermia is a serious and preventable health problem. Severe hypothermia (body temperature 30°C [86°F]) is associated with marked depression of critical body functions, which may make the victim appear clinically dead during the initial assessment.
Hypothermia affects virtually all organ systems. Perhaps the most significant effects are seen in the cardiovascular system and the CNS. Hypothermia results in decreased depolarization of cardiac pacemaker cells, causing bradycardia. Since this bradycardia is not vagally mediated, it can be refractory to standard therapies such as atropine. Mean arterial pressure and cardiac output decrease, and an electrocardiogram (ECG) may show characteristic J or Osborne waves
(Osborn waves are positive deflections occurring at the junction between the QRS complex and the ST segment, where the S point, also known as the J point, has a myocardial infarction-like elevation.
Sinus bradycardia tends to give way to AF leading to VF and asystole.
Arrhythmias other than VF tend to revert spontaneously as body temperature increases.
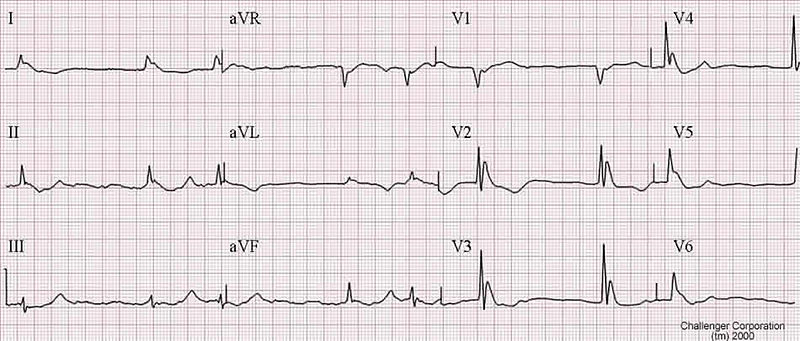
An example of an Osborn J wave in a hypothermic person. Source:http://askdrwiki.com/mediawiki/index.php?title=Image:Hypothermia.jpg; Author:WikiSysop
If the hypothermic victim has no signs of life, CPR should be performed without delay. If VT or VF is present, defibrillation should be attempted. If VT or VF persists after a single shock, the value of deferring subsequent defibrillations until a target temperature is achieved (>300C) is uncertain. It may be reasonable to perform further defibrillation attempts according to the standard ACLS / BLS algorithm concurrent with rewarming strategies (Class IIb, LOE C).
To prevent further loss of core heat, remove wet garments and protect the victim from additional environmental exposure. This should be done while providing initial BLS therapies. Rewarming should be attempted when feasible.
Warming:
ACLS management of cardiac arrest due to hypothermia focuses on aggressive active core rewarming techniques as the primary therapeutic modality. Patients with severe hypothermia and cardiac arrest can be rewarmed most rapidly with cardiopulmonary bypass if that is feasible.
Alternative effective core rewarming techniques include warm-water lavage of the thoracic cavity and extracorporeal blood warming with partial bypass using extracorporeal membrane oxygenation (ECMO); (rewarming the patient rapidly can be achieved with a bypass in which a heat exchanger and oxygenator are used with cannulation via the left femoral artery and vein).
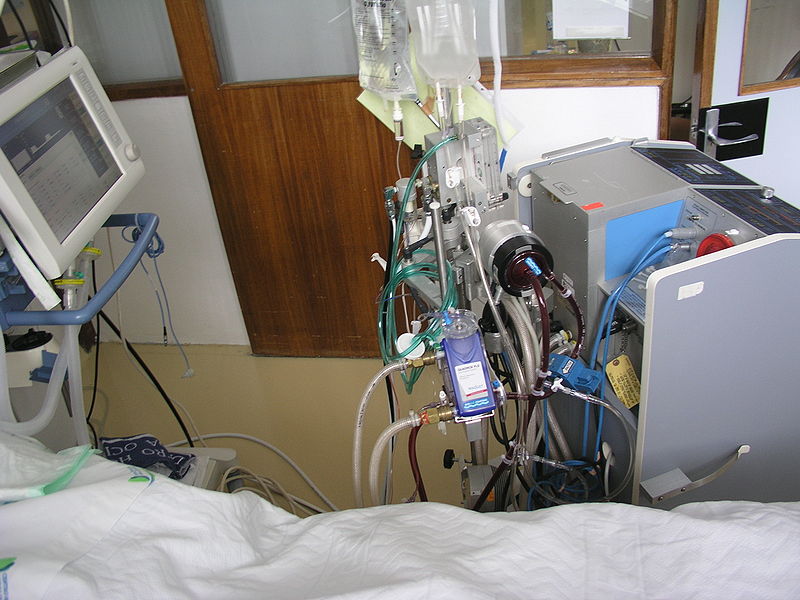
Extracorporeal membrane oxygenation (ECMO)
Adjunctive core rewarming techniques include warmed IV or intraosseous (IO) fluids and warm humidified oxygen. Heat transfer with these measures is not rapid and should be considered supplementary to active warming techniques.
Ventilation:
Advanced airway insertion is appropriate at this stage and recommended. Advanced airway management enables effective ventilation with warm humidified oxygen and reduces the likelihood of aspiration.
Effects of hypothermia:
Expectedly, the hypothermic heart may be unresponsive to cardiovascular drugs, pacemaker stimulation, and defibrillation; however, the data to support this are essentially theoretical. In addition, drug metabolism may be reduced, and there is a theoretical concern that medications could accumulate to toxic levels in the peripheral circulation if given repeatedly to the severely hypothermic victim.
For these reasons, previous guidelines suggest withholding IV drugs if the victim’s core body temperature is 30°C (86°F) or less. It may be reasonable to consider administration of a vasopressor during cardiac arrest according to the standard ACLS algorithm concurrent with rewarming strategies (Class IIb, LOE C).
ROSC:
After ROSC, patients should continue to be warmed to a goal temperature of approximately 32° to 34°C; this can be maintained according to standard postarrest guidelines for mild to moderate hypothermia in patients for whom induced hypothermia is appropriate (those who have ROSC but are still unconscious). For those with contraindications to induced hypothermia, rewarming can continue to normal temperatures.
Because severe hypothermia is frequently preceded by other disorders (eg, drug overdose, alcohol use, or trauma), the clinician must consider these underlying conditions while simultaneously treating hypothermia.
Patients with severe accidental hypothermia and cardiac arrest may benefit from resuscitation even in cases of prolonged downtime and prolonged CPR. Patients should not be declared dead before warming has been provided.
Temperature classification
Drowning
Each year drowning is responsible for more than 500 000 deaths worldwide1.
All victims of drowning who require any form of resuscitation (including rescue breathing alone) should be transported to the hospital for evaluation and monitoring, even if they appear to be alert and demonstrate effective cardiorespiratory function at the scene (Class I, LOE C).
Although survival is uncommon in victims who have undergone prolonged submersion and require prolonged resuscitation, successful resuscitation with full neurological recovery has occurred occasionally after prolonged submersion in icy water2-4. For this reason, scene resuscitation should be initiated and the victim transported to the nearest hospital unless there is obvious death (eg, rigor mortis, decomposition, hemisection, decapitation, lividity).
1. Peden MM, McGee K. The epidemiology of drowning worldwide. Inj Control Saf Promot. 2003;10:195–199
2. Southwick FS, Dalglish PH Jr. Recovery after prolonged asystolic cardiac arrest in profound hypothermia: a case report and literature review. JAMA. 1980;243:1250 –1253.
3. Siebke H, Rød T, Breivik H, Lind B. Survival after 40 minutes’ submersion without cerebral sequelae. Lancet. 1975;1:1275–1277.
4. Gilbert M, Busund R, Skagseth A, Nilsen PÅ, Solbø JP. Resuscitation from accidental hypothermia of 13.7°C with circulatory arrest. Lancet. 2000;355:375–376.
BLS / ACLS Modifications
The most important and detrimental consequence of submersion is hypoxia; therefore, oxygenation, ventilation, and perfusion should be restored as rapidly as possible. This will require immediate bystander CPR plus activation of the EMS system.
With the 2010 AHA Guidelines for CPR and ECC, CPR now begins with chest compressions. However, the guidelines recommend that healthcare providers tailor the sequence based upon the presumed etiology of the arrest. Healthcare provider CPR for drowning victims should use the traditional A-B-C approach in view of the hypoxic nature of the arrest (if no breathing, ventilate up to 5 times initially).
Recovery of the victim from water
When attempting to rescue a drowning victim, the rescuer should get to the victim as quickly as possible. It is crucial, however, that the rescuer pays constant attention to his or her own personal safety during the rescue process.
The reported incidence of cervical spine injury in drowning victims is low (0.009%)1-2.Unnecessary cervical spine immobilization can impede the adequate opening of the airway and delay delivery of rescue breaths. Routine stabilization of the cervical spine in the absence of circumstances that suggest a spinal injury is not recommended (Class III, LOE B).
1. Weinstein MD, Krieger BP. Near-drowning: epidemiology, pathophysiology, and initial treatment. J Emerg Med. 1996;14:461– 467.
2. Watson RS, Cummings P, Quan L, Bratton S, Weiss NS. Cervical spine injuries among submersion victims. J Trauma. 2001;51:658–662.
Rescue Breathing
The first and most important treatment of the drowning victim is the immediate provision of ventilation. Prompt initiation of rescue breathing increases the victim’s chance of survival1. Rescue breathing is usually performed once the unresponsive victim is in shallow water or out of the water.
As soon as the unresponsive victim is removed from the water, the rescuer should open the airway, check for breathing, and if there is no breathing, give 2 rescue breaths that make the chest rise (if this was not done previously in the water).
Some victims do not aspirate water because they develop laryngospasm or breath-holding2. Even if water is aspirated, there is no need to clear the airway of aspirated water, because only a modest amount of water is aspirated by the majority of drowning victims, and aspirated water is rapidly absorbed into the central circulation2. Attempts to remove water from the breathing passages by any means other than suctioning (eg, the Heimlich maneuver) are unnecessary and potentially dangerous.
1. Modell JH, Davis JH. Electrolyte changes in human drowning victims. Anesthesiology. 1969;30:414–420.
2. Kyriacou DN, Arcinue EL, Peek C, Kraus JF. Effect of immediate resuscitation on children with submersion injury. Pediatrics. 1994;94(pt 1):137–142.
Chest Compressions and Defibrillation
After delivery 2 effective breaths, if a pulse is not definitely felt, the healthcare provider should begin chest compressions and provide cycles of compressions and ventilation according to the BLS guidelines.
rescuers should attach an AED and attempt defibrillation if a shockable rhythm is identified. It is only necessary to dry the chest area before applying the defibrillation pads and using the AED. If hypothermia is present, follow the recommendations in: “Cardiac Arrest in Hypothermia.”
Vomiting
The drowning victim may vomit when the rescuer performs chest compressions or rescue breathing. In a 10-year study in Australia, two-thirds of victims who received rescue breathing and 86% of those who required compressions and ventilations vomited1. If vomiting occurs, turn the victim to the side and remove the vomitus using your finger, a cloth, or suction. If a cervical injury is suspected, the victim should be logrolled so that the head, neck, and torso are turned as a unit to protect the cervical spine.
1. Manolios N, Mackie I. Drowning and near-drowning on Australian beaches patrolled by life-savers: a 10-year study, 1973–1983. Med J Aust. 1988;148:165–167, 170–171.
Electrolyte abnormalities
Electrolyte abnormalities may be associated with cardiovascular emergencies and may cause or contribute to cardiac arrest, hinder resuscitative efforts, and affect hemodynamic recovery after cardiac arrest.
Early consideration may be given to using selective methods of therapeutic management in addition to standard ACLS protocols that can be provided rapidly and during resuscitation and have been shown to be effective in patients with cardiovascular instability.
Potassium (K)
Potassium is maintained mainly in the intracellular compartment through the action of the Na/K-ATPase pump which needs energy to function properly. The magnitude of the potassium gradient across cell membranes determines excitability of nerve and muscle cells, including the myocardium.
Potassium is tightly regulated. Under normal conditions, potential differences across membranes, especially cardiac, are not affected by alterations in potassium level. Rapid or significant changes in serum concentrations of potassium result from the large shifts of potassium from one space to another may have life-threatening consequences.
Hyperkalemia
Hyperkalemia is one of the few potentially lethal electrolyte disturbances. Severe hyperkalemia (defined as a serum potassium concentration 6.5 mmol/L) occurs most commonly from renal failure or from the release of potassium from cells (Crush injuries) and can cause cardiac arrhythmias and cardiac arrest. Iatrogenic potassium administration may be an additional cause.
Severe hyperkalemia may cause:
- flaccid paralysis,
- paresthesia,
- depressed deep tendon reflexes,
- respiratory difficulties,
The first indicator of hyperkalemia may be the presence of:
- peaked T waves (tenting) on the electrocardiogram (ECG).
- As serum potassium rises, the ECG may progressively develop flattened or absent P waves, a prolonged PR interval, widened QRS complex, deepened S waves, and merging of S and T waves.
- If hyperkalemia is left untreated, a sine-wave pattern, idioventricular rhythms, and asystolic cardiac arrest may develop.

ACLS Modifications in Management of Severe Cardiotoxicity or Cardiac Arrest Due to Hyperkalemia
Treatment of severe hyperkalemia aims at protecting the heart from the effects of hyperkalemia by antagonizing the effect of potassium on excitable cell membranes, forcing potassium into cells to remove it promptly from the circulation, and removing potassium from the body. Therapies that shift potassium will act rapidly but are temporary and thus may need to be repeated.
In order of urgency, treatment includes the following during resuscitation:
Stabilize myocardial cell membrane:
Calcium chloride (10%): 5 to 10 mL (500 to 1000 mg) IV over 2 to 5 minutes or calcium gluconate (10%): 15 to 30 ml IV over 2 to 5 minutes
Shift potassium into cells:
- Sodium bicarbonate: 50 mEq IV over 5 minutes
- Glucose plus insulin: mix 25 g (50 mL of D50) glucose and 10 U regular insulin and give IV over 15 to 30 minutes
- Nebulized albuterol: 10 to 20 mg nebulized over 15 minutes
Promote potassium excretion:
- Diuresis: furosemide 40 to 80 mg IV
- Kayexalate: 15 to 50 g plus sorbitol per oral or per rectum
- Dialysis
ACLS Modifications in Management of Severe Cardiotoxicity Due to Hypokalemia
Life-threatening hypokalemia is uncommon but can occur in the setting of gastrointestinal and renal losses and is associated with hypomagnesemia. Severe hypokalemia will alter cardiac tissue excitability and conduction. Hypokalemia can produce ECG changes such as:
- U waves,
- T-wave flattening,
- arrhythmias (especially if the patient is taking digoxin), particularly ventricular arrhythmias, which, if left untreated, deteriorate to PEA or asystole.
The effect of bolus administration of potassium for cardiac arrest suspected to be secondary to hypokalemia is unknown and ill-advised (Class III, LOE C).
Sodium
Sodium is the major intravascular ion that influences serum osmolality. Sodium abnormalities are unlikely to lead to cardiac arrest, and there are no specific recommendations for either checking or treating sodium during cardiac arrest. Disturbances in sodium level are unlikely to be the primary cause of severe cardiovascular instability.
Magnesium (Mg)
Magnesium is an essential electrolyte and an important cofactor for multiple enzymes, including ATPase. Magnesium is necessary for the movement of sodium, potassium, and calcium into and out of cells and plays an important role in stabilizing excitable membranes. The presence of a low plasma magnesium concentration has been associated with poor prognosis in cardiac arrest patients.
Hypermagnesemia
Hypermagnesemia is defined as a serum magnesium concentration 2.2 mEq/L (normal: 1.3 to 2.2 mEq/L). Neurological symptoms of hypermagnesemia include:
- muscular weakness,
- paralysis,
- ataxia,
- drowsiness, and confusion.
Hypermagnesemia can produce vasodilation and hypotension. Extremely high serum magnesium levels may produce a depressed level of consciousness, bradycardia, cardiac arrhythmias, hypoventilation, and cardiorespiratory arrest.
ACLS Modifications in Management of Cardiac Arrest and Severe Cardiotoxicity Due to Hypermagnesemia
Administration of calcium (calcium chloride [10%] 5 to 10 mL or calcium gluconate [10%] 15 to 30 ml IV over 2 to 5 minutes) may be considered during cardiac arrest associated with hypermagnesemia (Class IIb, LOE C).
Hypomagnesemia
Hypomagnesemia, defined as a serum magnesium concentration 1.3 mEq/L, is far more common than hypermagnesemia. Hypomagnesemia usually results from decreased absorption or increased loss of magnesium from either the kidneys or intestines (diarrhea). Alterations in thyroid hormone function, certain medications (eg, pentamidine, diuretics, alcohol), and malnourishment can also induce hypomagnesemia.
ACLS Modifications in Management of Cardiac Arrest and Severe Cardiotoxicity Due to Hypomagnesemia
Hypomagnesemia can be associated with polymorphic ventricular tachycardia, including torsades de pointes, a pulseless form (polymorphic) of ventricular tachycardia. For cardiotoxicity and cardiac arrest, IV magnesium 1 to 2 g of MgSO4 bolus IV push is recommended (Class I, LOE C).
Calcium
Calcium abnormality as an etiology of cardiac arrest is rare.
However, empirical use of calcium (calcium chloride [10%] 5 to 10 mL OR calcium gluconate [10%] 15 to 30 ml IV over 2 to 5 minutes) may be considered when hyperkalemia or hypermagnesemia is suspected as the cause of cardiac arrest (Class IIb, LOE C).
P.E.
ACLS modifications in PE
Pulmonary embolism (PE) can result in cardiovascular collapse and cardiac arrest. Cardiac arrest caused by PE often presents as pulseless electric activity (PEA).
In patients with cardiac arrest and without suspected PE, routine fibrinolytic treatment given during CPR shows no benefit and is not recommended (Class III, LOE A).
In patients with cardiac arrest and presumed PE, however, the use of fibrinolytics during CPR may improve the patient’s chance of survival. Despite the potential to increase the risk of severe bleeding, fibrinolytics may improve survival to discharge and long-term neurological function in patients with presumed PE-induced cardiac arrest. Emergency echocardiography may be helpful in determining the presence of thrombus or PE.
In a small number of patients, percutaneous mechanical thromboembolectomy during CPR has been performed successfully. Surgical embolectomy has also been used successfully in some patients with PE-induced cardiac arrest.
Accelerated thrombus removal mechanisms
Rotational Mechanical
A fragmentation cage pulled through the vein macerates and strips thrombus from the vein walls (Trerotola Arrow; PA, USA)
Isolated pharmaco-mechanical thrombolysis
Balloons largely confine the treatment area, where a thrombolytic agent is dispersed through thrombus with a rotating wire (Trellis, Covidien; CA, USA)
Bernoulli's principle effect
High-powered jets fragment the thrombus into microscopic pieces (Angiojet ® , Medrad Interventional/Possis; PA, USA)
US-assisted catheter-directed thrombolysis
US waves partially fragment thrombus that can be attacked by a thrombolytic agent (EndoWave™/EkoSonic ® , Ekos ® , WA, USA)
Toxic Injestion
Poisoning causes damage at the the cellular level, desrupting the natural victim’s physiology1. This may be manifested in the form of altering the cellular receptor normal functions which can no longer support life. These changes are seen in
- Ion channels,
- Cell organelles,
- Cell chemical pathways.
1- Trestrail JH. Criminal Poisoning: Investigational Guide for Law Enforcement, Toxicologists, Forensic Scientists, and Attorneys. 2nd ed. Totowa, NJ: Humana; 2007.
BLS / ALS modifications
As with all patient in cardiac arrest, management of the patient with a toxic exposure begins with support of airway, breathing, and circulation. Cardiac arrest is managed in accordance with the current standards of BLS and ACLS. With few exceptions, there are no unique antidotes or toxin-specific interventions that are recommended during resuscitation from cardiac arrest.
Once ROSC (return of spontaneous circulation) is achieved, urgent consultation with a medical toxicology unit is recommended, as the postarrest management of the critically poisoned patient may benefit from a thorough understanding of the toxic agent and provision of appropriate management.
Consultation is also recommended early in the management of a patient with potentially life-threatening poisoning, when appropriate interventions might prevent deterioration to cardiac arrest.
Management
The majority of questions addressing cardiac arrest due to drug toxicity remain unanswered. Some recommendations are evidence-based, but most research in this area consists of case reports, small case series, animal studies, and pharmacokinetic studies in healthy volunteers. Virtually no toxicology research involves human cardiac arrest. Thus, many of these recommendations are based on expert consensus, and further research is needed to validate them.
There are no data to support the use of specific antidotes in the setting of cardiac arrest due to:
Opioid Toxicity
Benzodiazepines
Blockers
Calcium Channel Blockers
Digoxin and Related Cardiac Glycosides
Cocaine
For cyclic Antidepressants poisoning leading to C.A., administration of sodium bicarbonate maybe considered1 (Class IIb, LOE C).
[In non C.A. patients with cyclic antidepressants poisoning, therapeutic strategies are directed towards the expected cardiotoxicity by increasing serum sodium, increasing serum pH, or doing both simultaneously. The relative contributions of hypernatremia and alkalemia are controversial, but in clinical practice administration of hypertonic sodium bicarbonate solution (8.4% solution, 1 mEq/mL) is the trend. Sodium bicarbonate boluses of 1 mL/kg may be administered as needed to achieve hemodynamic stability (adequate mean arterial blood pressure and perfusion) and QRS narrowing (Class IIb, LOE C)].
For Local Anesthetic Toxicity, clinical case reports2,3 , and controlled animal studies4,5 have suggested that rapid IV infusion of lipids may reverse this toxicity either by redistributing the local anesthetic away from its site of action or by augmenting metabolic pathways within the cardiac myocyte. Case reports have shown return of spontaneous circulation in patients with prolonged cardiac arrest unresponsive to standard ACLS measures6,7.
It may be reasonable to consider 1.5 mL/kg of 20% long-chain fatty acid emulsion as an initial bolus, repeated every 5 minutes until cardiovascular stability is restored (Class IIb, LOE C).363 After the patient is stabilized, some papers suggest a maintenance infusion of 0.25 mL/kg per minute for at least 30 to 60 minutes. A maximum cumulative dose of 12 mL/kg has been proposed.
Carbon Monoxide Poisoning:
In addition in reducing the ability of hemoglobin to deliver oxygen, carbon monoxide causes direct cellular damage to the brain and myocardium8. Survivors of carbon monoxide poisoning are at risk for lasting neurological injury8. Several studies have suggested that very few patients who develop cardiac arrest from carbon monoxide poisoning survive to hospital discharge, regardless of treatment administered following return of spontaneous circulation9. Routine care of patients in cardiac arrest and severe cardiotoxicity from carbon monoxide poisoning should complywith standard BLS and ACLS recommendations.
Cyanide Poisoning:
Cyanide is a major component of fire smoke, and cyanide poisoning must be considered in victims of smoke inhalation who have hypotension, central nervous system depression, metabolic acidosis, or soot in the nares or respiratory secretions. Cyanide poisoning is known to cause rapid cardiovascular collapse, manifested as hypotension, lactic acidosis, central apnea, and convulsions.
Patients in cardiac arrest or those presenting with cardiovascular instability caused by known or suspected cyanide poisoning should receive 100% oxygen and cyanide-antidote with a cyanide scavenger (either IV hydroxocobalamin or a nitrate such as IV sodium nitrite and/or inhaled amyl nitrite), followed as soon as possible by IV sodium thiosulfate (Class I, LOE B).
Both hydroxocobalamin and sodium nitrite serve to rapidly and effectively bind cyanide in the serum and reverse the effects of cyanide toxicity. Hydroxocobalamin has a safety advantage over nitrites as the later induces methemoglobin formation and can cause hypotension, particularly in children and victims of smoke inhalation who might also have concurrent carbon monoxide poisoning.
Sodium thiosulfate acts as a metabolic cofactor, enhancing the detoxification of cyanide to thiocyanate. Thiosulfate administration enhances the effectiveness of cyanide scavengers and has been used successfully in humans with both hydroxocobalamin sodium nitrite.
Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9:588 –590.
Foxall GL, Hardman JG, Bedforth NM. Three-dimensional, multiplanar, ultrasound-guided, radial nerve block. Reg Anesth Pain Med. 2007;32: 516–521.
Shah S, Gopalakrishnan S, Apuya J, Martin T. Use of Intralipid in an infant with impending cardiovascular collapse due to local anesthetic toxicity. J Anesth. 2009;23:439–441.
Cave G, Harvey MG, Winterbottom T. Evaluation of the Association of Anaesthetists of Great Britain and Ireland lipid infusion protocol in bupivacaine induced cardiac arrest in rabbits. Anaesthesia. 2009;64: 732–737.
Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88:1071–1075.
Litz RJ, Popp M, Stehr SN, Koch T. Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia. 2006;61:800–801.
Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105: 217–218.
Weaver LK. Clinical practice: carbon monoxide poisoning. N Engl J Med. 2009;360:1217–1225.
Hampson NB, Zmaeff JL. Outcome of patients experiencing cardiac arrest with carbon monoxide poisoning treated with hyperbaric oxygen. Ann Emerg Med. 2001;38:36–41.