Medications
- Adrenaline
- Amiodarone
- Aspirin
- Atropine
- Adenosine
- Beta-adrenergic blocking agents
- Ca channel blockers
- Dopamine
- Dobutamine
- Magnesium
- Nitroglycerine
- Tenecteplase (Metalyse)

Adrenaline
Indications Dose
Cardiac arrest of any cause Second-line treatment for cardiogenic shock.
As an alternative to external pacing for bradycardia. | 1 mg IV / IO every 3-5 min
0.05–1 mcg kg-1 min-1
2–10 mcg min-1 |
Anaphylaxis 0.3-0.5mg IM or 50-100mcg IV in severe cases
Uses
Adrenaline is available in two dilutions:
· 1 in 10 000 (10 ml contains 1 mg of adrenaline).
· 1 in 1 000 (1 ml contains 1 mg of adrenaline).
During cardiac arrest, if intravascular (IV) access is difficult to be achieved, the IO route is now recommended as an alternative with the same IV dose.
Following the return of spontaneous circulation (ROSC), adrenaline may induce various arrhythmias like tachycardia (ventricular tachycardia (VT) and ventricular fibrillation (VF) which may lead to myocardial ischaemia. Once a perfusing rhythm is established, and if further adrenaline is required when other inotropic drugs (such as dobutamine) have failed to improve the cardiac output it is then given by infusion, 0.05-1 mcg kg-1 min-1. Start the infusion at a low rate and titrate to the mean arterial pressure and/or cardiac output.
Adrenalin is also indicated for symptomatic bradycardia which has not responded to atropine, and external pacing is unavailable or unsuccessful. The dose range is usually 2–10 mcg min-1.
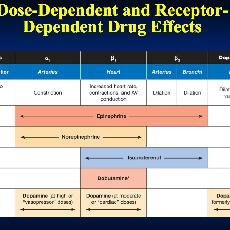
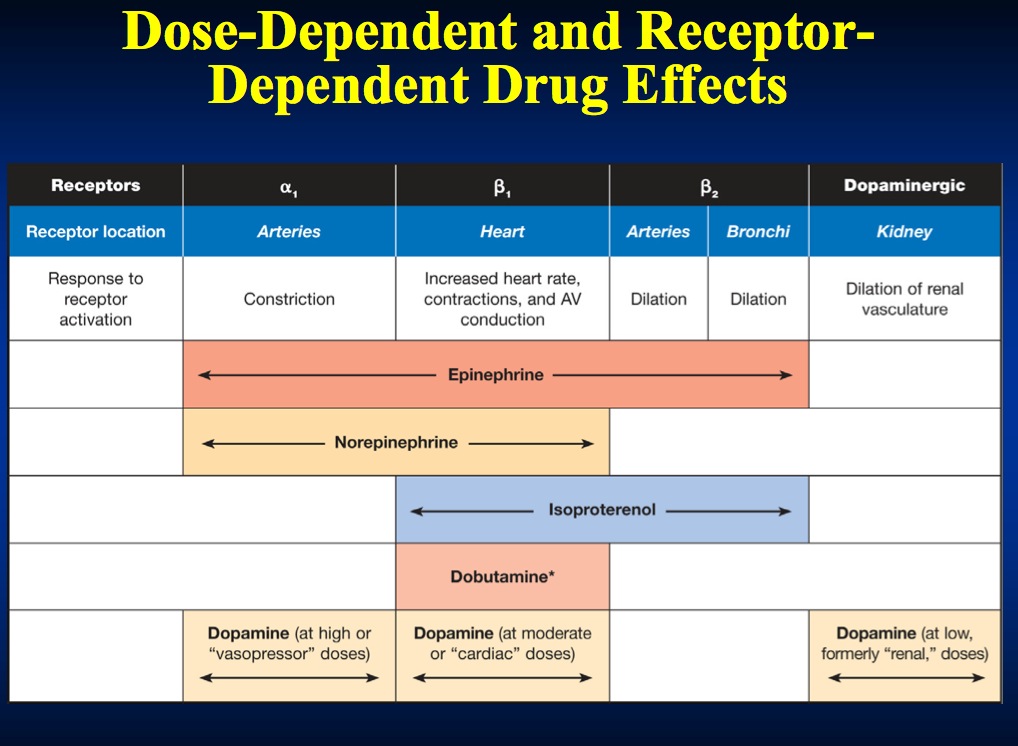
Adrenaline is a direct-acting sympathomimetic amine that possesses both alpha (α) - and beta (β) adrenergic activity. In the dose used in resuscitation, adrenaline stimulates both alpha (α) and beta (ß) receptors to produce peripheral vasoconstriction. This increases systemic vascular resistance (SVR) during CPR, and improves both cerebral and coronary perfusion pressures.
In the beating heart, the action of adrenaline on β1 receptors increases heart rate and force of contraction. This increases myocardial oxygen consumption which may worsen ischaemia. Adrenaline increases myocardial excitability and is therefore potentially arrhythmogenic, especially during myocardial ischaemia. Adrenaline may also cause GIT ischaemia.
|
Adrenaline 1:1000 & 1:10,000 |
Adrenaline mini jet |
Inotropic drugs |
Epipen, Child/Adult |
Amiodarone
Indications Dose
Refractory VF/ pulseless VT 300 mg IV
Control of haemodynamically stable VT, polymorphic VT, and broad-complex tachycardia of uncertain origin Paroxysmal SVT when adenosine, vagal manoeuvres, and AV nodal blockade are unsuccessful. To control rapid ventricular rate caused by accessory pathway conduction (WPW) in pre-excited atrial arrhythmias | 300 mg IV over 20-60 min followed by an infusion of 900mg over 24 h |
Use
Paradoxically amiodarone can be arrhythmogenic, especially if given together with other drugs that prolong the QT interval. But, it has a lower incidence of pro-arrhythmic effects compared to other antiarrhythmic agents if used under similar situations.
The main side effects are hypotension due to the solvent Polysorbate, which causes histamine release, and bradycardia, which may not be noticed till ROSC.
The side effects associated with prolonged oral use are photosensitivity, thyroid & hepatic dysfunction, peripheral neuropathy & pulmonary inflammation/fibrosis.
An initial intravenous bolus dose of 300 mg amiodarone, (may be diluted in 20ml 5% dextrose, and given over 20 seconds), is considered if VF/VT persists after the third shock.
If a peripheral vein is used, amiodarone may cause thrombophlebitis; a central vein is preferable otherwise use a large peripheral vein followed by a 20 ml flush.
For tachyarrythmias, amiodarone is given as 300 mg intravenously over 45-60 min. This loading dose is followed by an infusion of 900 mg over 24 h. Give additional doses of 150 mg as necessary for recurrent or resistant arrhythmias to a maximum total daily dose of 2 g.
In cases of severely impaired heart function, intravenous amiodarone is preferable to other antiarrhythmic drugs for atrial and ventricular arrhythmias. Hypotension and bradycardia can be prevented by slowing the rate of the infusion.
The plasma levels of warfarin and digoxin are increased by amiodarone; therefore their doses should be readjusted. INR for warfarin and digoxin levels should be monitored.
Amiodarone has an additive effect to that of beta-blockers and calcium channel blockers on the degree of AV nodal blockade and hypotension.
Actions:
Amiodarone is a membrane stabilising antiarrhythmic drug that increases the duration of both the refractory period and action potential in the myocardium.
AV conduction and accessory pathway conduction is slowed. Amiodarone has a very mild negative inotropic action and may cause peripheral vasodilation by non-competitive alfa blockade.
|
Amiodarone inj 150mg |
Aspirin
Indications Dose
Acute coronary syndromes 300-325 mg to be chewed followed by 75 mg daily orally
Use:
The oral dose is 160-300 mg to patients with acute coronary syndromes irrespective of the delay to the first evaluation of the patient.
Chronic aspirin usage may cause acute or chronic upper GI bleeding. The risk is dose-related. If thrombolytic therapy is given in the early phase, give aspirin concomitantly to help reduce the risk of early coronary artery re-occlusion.
Actions:
Aspirin improves the prognosis of patients with acute coronary syndromes and reduces mortality. The effect is achieved by anti-platelet activity (Irreversibly acetylates platelets COX1, thus inhibiting formation of thromboxane A2 preventing platelets adhesions.
Atropine
Indications Dose
Sinus, atrial, or nodal bradycardia when the patient is symptomatic 0.6mg increments IV to a maximum of 3 mg
Use:
For symptomatic bradycardia, give an initial dose of 0.6 mg IV. Repeated doses may be necessary, but consider pacing if this is ineffective.
Conduction disturbance or bradycardia associated with the high vagal activity may respond to atropine (e.g oculocardiac reflex).
There is no conclusive evidence that atropine is of any value in asystolic cardiac arrest.
Actions:
Atropine antagonizes the neurotransmitter acetylcholine at muscarinic receptors. Thus it blocks the effect of the vagus nerve on both the SA and AV nodes, increasing sinus automaticity and facilitating AV nodal conduction.
Side-effects of atropine are dose-related (blurred vision, dry mouth and urinary retention). Acute confusional states may occur following intravenous administration in elderly patients.
After cardiac arrest, do not attribute dilated pupils solely to atropine.
Adenosine
Indications Dose
Stable narrow-complex tachycardia (or broad-complex tachycardia known to be a supraventricular tachycardia (SVT) with bundle branch block) which is not responding to vagal manoeuvres. Note: While the limb is elevated, Adenosine must be injected rapidly IV followed immediately with 20ml isotonic saline flush. IV canulae should be inserted in the anticubital veins. It has a half life of 5-10 sec due to rapid enzymatic degradation (adenosine deaminase). | 6 mg, 12 mg, IV |
Use:
Adenosine should be administered in a monitored environment only (e.g. coronary care unit, critical care unit, operating room, or emergency department); it may cause a transient period of ventricular asystole.
It may be used as a diagnostic tool, and unlike verapamil, it can be given to a patient with a broad-complex tachycardia of uncertain aetiology. The ventricular rate in SVT will be slowed but ventricular tachycardia (VT) is unchanged.
Adenosine is effective in terminating the re-entry tachycardias that originate in the AV node (SVT). It does not have significant negative inotropic effects and does not cause decreased cardiac output and hypotension, and may be given safely with a beta-blockers on board.
Patients may encounter transient unpleasant side-effects: nausea, flushing, and chest discomfort, sense of impending doom and should be clearly explained to them.
Theophylline (aminophylline) and related compounds antagonize adenosine. Patients on dipyridamole or carbamazepine or those with denervated (transplanted) hearts display a markedly hazardous exaggerated effect.
Adenosine is contraindicated in patients with WPW syndrome, as the conduction blockade through the AV node by adenosine may promote conduction down the accessory pathway. In the presence of pre-excited atrial fibrillation or flutter, this may cause a dangerously rapid ventricular response.
The initial dose is 6 mg; if unsuccessful follow this with 12 mg after 1-2 min.
|
Adenosine |
Beta-adrenergic blocking agents
Indications Dose
Narrow-complex tachycardias uncontrolled by vagal manoeuvres and/or adenosine in the patient with preserved ventricular function. To control rate in AF and atrial flutter with < 48h duration and preserved ventricular function. | Atenolol (β1) 5 mg IV over 5 min Esmolol (β1) 500 mcg kg-1 over 1 min followed by an infusion of50- 200 mcg kg-1min-1 Metoprolol (β1) 2-5 mg IV at 5 min Propanolol (β1 and ß2) 100 mcg kg-1 in 3 divided doses at 2 min intervals |
Use:
A beta-blocker may be used as a second-line drug for the treatment of narrow-complex tachycardia (or broad complex tachycardia known to be an SVT with aberrant conduction), when adenosine fails.
They may precipitate LVF in patients with a failing ventricle, hypotension, bradycardia or even heart block. The risk of developing heart block or asystole is increased if verapamil is given intravenously to a beta-blocked patient. For similar reasons, avoid the combination of beta-blockers and other antiarrhythmic drugs such as lidocaine.
Esmolol is short-acting with a half-life of 2-9 min. It is a ß1-selective beta-blocker administered IV.
Actions:
Beta-blocking drugs block the effects of circulating catecholamines with a resultant decrease
in heart rate and blood pressure. They also have a proven cardioprotective effect in patients with acute coronary syndromes.
Side effects of beta-blockade include bradycardia, AV conduction delay, hypotension and bronchospasm.
Contraindicated in second- or third-degree heart block, hypotensive states, CHF and asthma.
Ca channel blockers
Stable narrow complex tachycardia that is not terminated by vagal manoeuvres or adenosin Control of ventricular rate in patients with AF or atrial flutter (< 48h) |
|
Use
IV verapamil may be used in the treatment of SVT only when the diagnosis has been confirmed.
It has a marked negative inotropic effect and must not be given in broad complex tachycardia of ventricular or doubtful origin.
As with other vasodilators, sde effects may include flushing, headaches and hypotension. The antiarrhythmic effect may persist for about 6 h after an IV dose.
The initial dose of verapamil is 2.5 mg IV given over 2 min. A dose of 5-10 mg may be repeated every 15-30 min to a maximum of 20 mg.
Diltiazem 0.25 mg kg-1 IV, followed by a second dose of 0.35 mg kg-1, is as effective as verapamil.
Verapamil, and to a lesser extent, diltiazem may decrease myocardial contractility and reduce cardiac output in patients with severe left ventricular dysfunction. It may be harmful when given to patients with pre-excited AF or atrial flutter associated with Wolff Parkinson White syndrome as
this may promote conduction through the accessory pathway causing dangerously high ventricular rates.
Actions
Verapamil and diltiazem are calcium channel blocking drugs that slow conduction and increase refractory period in the AV node. These actions may terminate re-entry arrhythmia that involves the AV node (e.g. SVT) and contributes to control of ventricular response rate in patients with other atrial tachycardias.
Dopamine
Indications Dose
Hypotension in the absence of hypovolaemia 1-10 mcg kg-1 min-1
Use
There is a marked individual variability in the response to dopamine. Dopamine increases urine volume but has no beneficial effect on renal function as such. Dopamine should be given via a
central vein, using an infusion pump. Its use requires invasive haemodynamic monitoring in an intensive care or high-dependency care area.
Actions
Dopamine is the precursor of the naturally-occurring catecholamines adrenaline and noradrenaline. It has a dose-dependent positive inotropic effect that is mediated by dopamine (D1 and D2), α1- and β1- receptors.
Theoretically, low infusion rates (1-2 mcg kg-1 min-1) cause renal artery vasodilation (via D1 receptors), and increased glomerular filtration rate and sodium excretion.
Intermediate infusion rates (2-10 mcg kg-1 min-1) increase cardiac output and systolic blood pressure (β1). With higher infusion rates (> 10 mcg kg- 1 min-1) α1- and α2- effects dominate causing vasoconstriction.
Dopamine may cause cardiac arrhythmias, increase myocardial oxygen demand and worsen ischaemia. Dopamine and dobutamine may be administered together and titrated to effect.
Dobutamine
Indications Dose
Hypotension not caused by hypovolaemia 5-20 mcg kg-1 min-1
Cardiogenic shock
Use
Dobutamine is the inotropic drug of choice in the post-resuscitation period. It is indicated in poor cardiac output states with compromised tissue perfusion. It is useful in pulmonary oedema associated with a low BP which contraindicates the use of other vasodilators.
Close haemodynamic monitoring of the patient is essential and should be undertaken in a high-dependency or intensive care area. Tachycardia and cardiac arrhythmias may occur, particularly at higher doses. Reduce the infusion slowly to avoid hypotension.
The drug has a short half-life and is given by intravenous infusion using an infusion pump. The dose range is 5-20 mcg kg-1 min-1 titrated to effect.
Actions
A synthetic catecholamine with ß1 (positive inotropic effect on myocardium) ß2,( peripheral vasodilatation) and α1 -receptors stimulant effect. The net effect is to increase cardiac output. Renal blood flow is usually increased.
Dobutamine increases myocardial oxygen requirements less than other inotropic agents and is less arrhythmogenic.
Magnesium
Indications Dose
Shock refractory ventricular fibrillation in the presence of 2 g bolus IV
hypomagnesaemia
Ventricular tachyarrhythmias in the presence of hypomagnesaemia 2 g over 10
min IV
Torsade de pointes
Intravenous magnesium is a safe and often effective treatment for ventricular tachyarrhythmias as Torsades de pointes.
Magnesium is excreted by the kidneys. It inhibits smooth muscle contraction, leading to vasodilation, and dose-related hypotension; this is a transient effect which normally responds to intravenous fluids and vasopressors.
Actions
Magnesium is a major constituent of many enzymes in the body, especially those needed for the production of ATP in the muscle cells. It inhibits acetylcholine release and reduces the sensitivity of the motor end plate. The normal plasma level of magnesium is 0.80–1.00 mmol l-1.
Magnesium deficiency frequently co-exists with other electrolyte disturbances, particularly hypokalaemia. Thus digoxin toxicity may be manifested even within the therapeutic digoxin level.
Nitroglycerine
Indications:
- Administer to all patients with suspected ischemic pain unless contraindicated
- Remember to reassess and repeat vital signs between doses
- Up to 3 sublingual or spray doses may be given at 5-minute intervals
- IV initiated for specific indications
Actions:
- Decreases pain of ischemia
- Increases venous dilation
- Decreases venous blood return to heart (pre-load)
- Decreases preload and cardiac oxygen consumption
- Dilates coronary arteries
- Increases cardiac collateral flow IV Nitroglycerin STEMI Indications:
First 24 to 48 hours in patients with STEMI and one or more of the following:
- Recurrent ischemic chest pain
- LV failure (acute pulmonary edema or CHF)
- Elevated BP (especially with signs of LV failure)
- Large anterior infarction
- Persistent ischemia
Dose:
- Sublingual:0.3to0.4mg;repeat every 5 minutes
Sprayinhaler:2metered doses at 5-minute intervals
- IV infusion:12.5 to 25μg bolus, 10 to 20 μg/min infusion, titrated
Precautions:
- Contraindicated if systolic BP
- Contraindicated in RV infarction
- Suspect RV infarction with inferior ST changes
- Limit BP drop to 10% if patient is normotensive
- Limit BP drop to 30% if patient is hypertensive
- Watch for a headache, drop in BP, syncope, tachycardia
- Tell patient to sit or lie down during administration
Key Concept:
Careful assessment of the patient before and during nitrate administration is necessary. Hypotension in ACS should be avoided because a drop in mean coronary perfusion pressure can propagate thrombus and increase infarct size.
Additional contraindications to nitrates:
• Recent phosphodiesterase inhibitor use. If the patient has erectile dysfunction and has taken a phosphodiesterase inhibitor within the previous 24 hours (48 hours for tadalafil), nitrates may cause severe hypotension refractory to vasopressor agents.
• Hypotension, bradycardia, or tachycardia. Avoid use of nitroglycerin in patients with hypotension (systolic BP 100 bpm).
• RV Infarction. Nitroglycerin should be used with caution in patients with inferior wall MI with possible RV involvement. Patients with RV dysfunction and acute infarction are very dependent on maintenance of RV filling pressures to maintain cardiac output and blood pressure.
• Transdermal preparations are relatively contraindicated in potentially unstable patients; topical application results in variability in the amount of drug delivered, and absorption is often erratic. Avoid long-acting oral preparations.
Tenecteplase (Metalyse)
Indication
For use in mortality reduction associated with acute myocardial infarction (AMI). Treatment should be initiated as soon as possible after the onset of AMI symptoms.
Mechanism of Action
Tenecteplase is a recombinant fibrin-specific plasminogen activator that is derived from native t-PA by modifications at three sites of the protein structure. It binds to the fibrin component of the thrombus (blood clot) and selectively converts thrombus-bound plasminogen to plasmin, which degrades the fibrin matrix of the thrombus. Tenecteplase has a higher fibrin specificity and greater resistance to inactivation by its endogenous inhibitor (PAI-1) compared to native t-PA.
Pharmacokinetics
Half-Life: 90-130 min
Onset: 30 min
Metabolism: Liver
Metabolites: Degradation products (constituent amino acids of tenecteplase)
Excretion: Clearance: Plasma: 99-119 mL/min
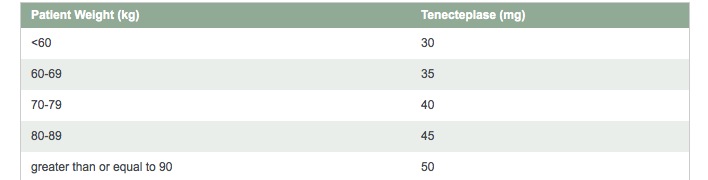
Dose
Single bolus tenecteplase features a simple, 5-tiered weight-based dosing schedule—tailored to the patientTenecteplase is for IV administration only
The recommended total dose should not exceed 50 mg
FLUSH a dextrose-containing line with a saline-containing solution prior to and following administration (precipitation may occur when tenecteplase is administered in an IV line containing dextrose). ADMINISTER as an IV BOLUS over 5 seconds.
Important Safety Information
Tenecteplase therapy in patients with acute myocardial infarction is contraindicated in the following:
- active internal bleeding,
- history of cerebrovascular accident,
- known bleeding diathesis,
- severe uncontrolled hypertension) because of an increased risk of bleeding
The most common complication encountered during tenecteplase therapy is bleeding. Should serious bleeding (not controlled by local pressure) occur, any concomitant heparin or antiplatelet agents should be discontinued immediately?
In certain conditions (eg, recent major surgery, cerebrovascular disease, hypertension) the risk of tenecteplase therapy may be increased and should be weighed against the anticipated benefits.
Cholesterol embolism has been reported rarely in patients treated with all types of thrombolytic agents; the true incidence is unknown.
Coronary thrombolysis may result in arrhythmias associated with reperfusion. It is recommended that anti-arrhythmic therapy for bradycardia and/or ventricular irritability be available when Tenecteplase is administered.
Reperfusion arrhythmias are common and include:
Bradycardia complete & heart block occurs most commonly with reperfusion of inferior MI. Usually, it resolves within minutes. If necessary treat with Atropine and fluids.
Non-sustained VT: runs are common and usually subside with time. Observe for 10 minutes before contemplating antiarrhythmics. Keep Potassium between 4-5 mmol/L.
Sustained VT / VF: Defibrillation and antiarrhythmics (including IV Mg)
How supplied:
Tenecteplase is supplied as a sterile, lyophilized powder in vials under partial vacuum. Each vial of tenecteplase is packaged with one vial of Sterile Water for Injection, USP for reconstitution.
Stability and Storage
Store lyophilized tenecteplase at controlled room temperature not to exceed 30°C (86°F) or under refrigeration 2–8°C (36–46°F). Do not use beyond the expiration date stamped on the vial.