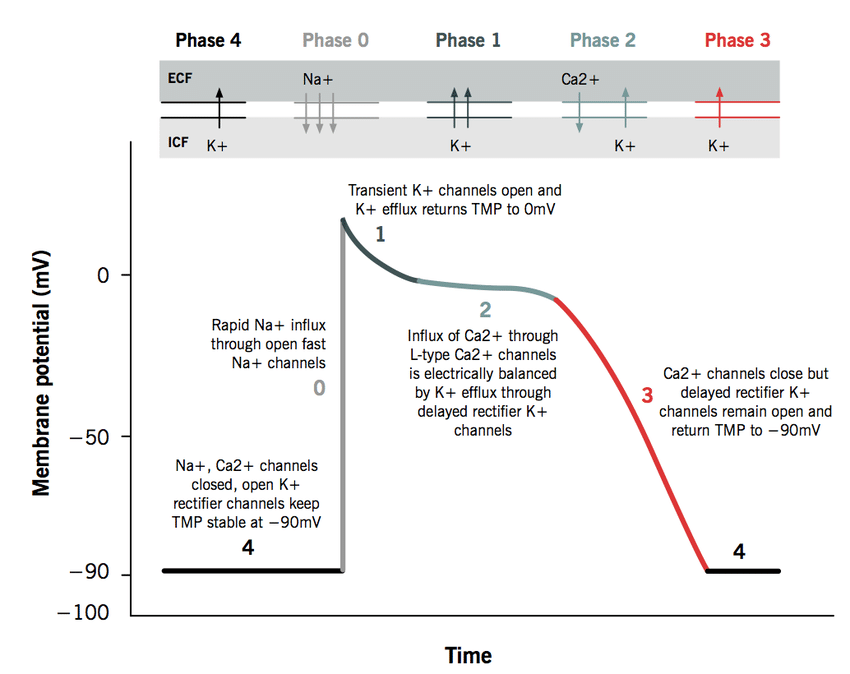
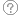Physiology (optional)
Autonomic innervation of the heart
The medulla, located in the brainstem is the primary site in the brain for regulating both sympathetic and parasympathetic (vagal) outflow to the heart and blood vessels.
The nucleus tractus solitarius (NTS) of the medulla receives sensory input from different systemic and central receptors (e.g., baroreceptors and chemoreceptors). The medulla also receives information from other brain regions (e.g., hypothalamus).
The hypothalamus and higher centres modify the activity of the medullary centres and are particularly important in stimulating cardiovascular responses to emotion and stress (e.g., fear, exercise).
Autonomic outflow fibres from the medulla are divided principally into:
- sympathetic
- parasympathetic (vagal).
Efferent fibres of these autonomic nerves travel to the heart and blood
vessels to modulate their activities.
The heart is innervated by parasympathetic (vagal) and sympathetic fibres.
The right vagus nerve primarily innervates the SA node, whereas the left
vagus innervates the AV node. The atrial muscle is also innervated by vagal
efferents.
Sympathetic efferent innervates the atria (especially in the SA node) and
ventricles, including the conduction system of the heart.
Rapid heartbeat patterns
Mechanisms of cardiac arrhythmias: from automaticity to re-entry
(reentry)
Mechanisms of cardiac arrhythmias:
automaticity to re-entry
Source: https://ecgwaves.com/topic/mechanisms-cardiac-arrhythmias-automaticity-reentry-triggered-activity/
The aim of this review is to present the most common arrhythmias in clinical practice. The initial discussion will focus on mechanisms of cardiac arrhythmias.
Although a detailed understanding of the mechanisms underlying cardiac arrhythmias is not necessary for all clinicians, it is wise to invest time in grasping the underlying concepts.
I will begin this review with a discussion on arrhythmogenesis (mechanisms of arrhythmias) and thereafter each arrhythmia is discussed separately.
It is appropriate to subdivide cardiac arrhythmias into the following groups:
Bradyarrhytmias (bradycardia): arrhythmias that are typically due to dysfunctional automaticity in pacemaker cells or blocking of impulses somewhere along the conduction system.
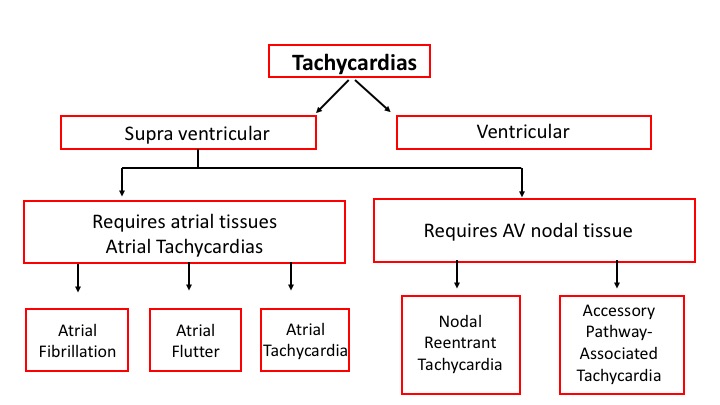
Supraventricular tachyarrhythmias (tachycardia): arrhythmias in which the impulses emanate from the atria.
Ventricular tachyarrhythmias (tachycardia): arrhythmias in which the impulses emanate from the ventricles.
The recommendations presented throughout this
section is in line with guidelines and recommendations issued by the European Society for Cardiology (ESC), the American Heart Association (AHA) and the American College of Cardiology (ACC).
Definition of heart rhythm
A rhythm is defined as three consecutive heart beats displaying identical waveforms on the ECG.
The similarity of the waveforms indicates that the
origin of the impulse is the same. The sinoatrial (SA) node is the heart’s pacemaker under normal circumstances and the rhythm is referred to
as sinus rhythm.
An arrhythmia is defined as an abnormal heart rhythm or heart rate, which is not physiologically justified.
The latter criteria is important because rhythms which are physiologically justified should not be considered abnormal. For example, sinus bradycardia is a common finding in athletes and during sleep; in these scenarios it should not be considered abnormal.
On the other hand, sinus bradycardia developing during physical exercise is considered abnormal, because heart rate must increase during exercise.
Mechanisms of cardiac arrhythmias
The mechanisms underlying cardiac arrhythmias are being unravelled at an increasing pace. Arrhythmology is a very exciting area with intense research activity. This is partly due to the rise of cardiac imaging and
invasive electrophysiological methods which enable detailed in vivo studies of arrhythmias.
Main causes of cardiac arrhythmias
Arrhythmias arise if the impulse formation is abnormal, or if the impulse transmission is abnormal, or if both these are abnormal.
These circumstances are now discussed in detail.
Abnormal impulse formation
Abnormal impulse formation can cause arrhythmias by the following two mechanisms:
- Increased or abnormal automaticity
- Triggered activity
Increased or abnormal automaticity
There are several structures in the heart that possess automaticity (i.e., ability to depolarise spontaneously).
These structures are as follows:
- The sinoatrial (SA) node: the sinoatrial node is the primary pacemaker of the heart. It directs the heart rhythm during normal circumstances and the rhythm is referred to as sinus rhythm.
- Parts of the atrial myocardium: There are clusters of atrial myocardial cells located around the crista terminalis, the entrance of the coronary sinus and the inferior vena cava, as well as cells around the mitral and tricuspid valves, which possess automaticity.
These cells are not conduction cells per se; they are actually contractile cells which possess automaticity. Thus, automaticity is not exclusive to cells of the conduction system.
- Myocardium surrounding the atrioventricular (AV) node: It is a common misconception that the atrioventricular (AV) node possesses automaticity, because there is no compelling evidence for that. There is, however, evidence that cell clusters surrounding the AV node possess automaticity. This automaticity will still – despite what has just been stated – be referred to as automaticity of the AV node in order to facilitate understanding.
- The His-Purkinje network: The bundle of His and the entire Purkinje network possess automaticity.
These are the natural pacemakers of the heart, because these structures possess automaticity, which is the intrinsic ability to depolarize spontaneously without previous stimulation.
The intrinsic rate of spontaneous depolarization in these pacemaker structures follows:
- Sinoatrial node: 70 depolarizations per minute.
- Atrial myocardium: 60 depolarizations per minute.
- Cells around the atrioventricular node: 40 depolarizations per minute
- His-Purkinje network: 20–40 depolarizations per minute.
The reason that the sinoatrial node is the primary pacemaker is simply because it has the fastest automaticity. Heart rhythm is directed by the
fastest pacemaker because that pacemaker will depolarize before the competing pacemakers and reset their “clocks” before they discharge an action potential. The list also indicates that automaticity diminishes gradually with the distance from the sinoatrial node. This stepwise decline in automaticity is referred to as the pacemaker hierarchy of the heart.
The sinoatrial node may become dysfunctional and fail to depolarise. This could potentially result in cardiac arrest but it rarely does, because absence of sinoatrial impulses will allow for one of the other pacemakers to take over the heart rhythm. This behaviour is the reason why the other pacemakers are often referred to as latent pacemakers. Any rhythm that replaces the sinus rhythm is referred to as an escape rhythm. In case the sinoatrial node is dysfunctional, an escape rhythm will most likely come from atrial myocardium, because it has the second highest rate of spontaneous depolarization. If the atrial myocardium also fails to generate action potentials, it is likely that an escape rhythm will come from cells around the atrioventricular node and so on.
Note that ventricular myocardium does not possess automaticity.
The automaticity in the sinoatrial node increases during physical exercise. The increased automaticity is a normal reaction since the cardiac output must increase during exercise. This is an example of normal (physiological) increase in automaticity. However, in certain circumstances the automaticity in the sinoatrial node and the other latent pacemakers can increase without physiological motivation. Some examples follow:
- Automaticity in the sinoatrial node can increase without physiological motivation and cause sinus tachycardia at rest. This is called inappropriate sinus tachycardia.
- The automaticity in latent pacemakers can increase, for example, during hypoxia, whereby they start discharging action potentials at higher rate than the sinoatrial node and thus take over the heart rhythm.
- Purkinje cells located around the ischemic zone during acute myocardial ischemia/infarction can increase their automaticity and initiate ventricular tachycardia.
Triggered activity (after depolarizations)
An action potential may induce an after depolarization, which is a depolarization occurring either during or after the repolarization phase.
An after depolarization occurring during repolarization is referred to as an early depolarization, whereas after depolarizations occurring after the repolarization are referred to as late depolarizations.
Early and late depolarizations may be strong enough to reach the threshold for eliciting another depolarization. In other words, after depolarizations may trigger action potentials. An action potential which is engendered by an after depolarization is referred to as a triggered action potential. Such action potentials cause extra systoles (extra heart beats that fall in between the normal beats).
Early depolarizations are typically seen during bradycardia, hypokalaemia, hypoxia, acidosis, hypocalcaemia and in drug side effects.
Late depolarizations are seen in digoxin overdosing and during sympathetic stimulation.
Importantly, after depolarizations may cause extra systoles but they do not cause persistent arrhythmias. However, the extra systoles might induce another arrhythmia mechanism (re-entry, see below) which may cause persistent arrhythmias.
Abnormal impulse conduction: re-entry (re-entry)
Normal impulse transmission implies that the depolarizing wave spreads rapidly, uniformly and unhindered through the myocardium. This requires
that all cells ahead of the impulse wave are excitable and offer equal capacity to transmit the impulse. Only under such circumstances can the depolarization (the impulse) spread through the myocardium like a wave
front in water. Should the impulse encounter cells that are not excitable or areas where the conductivity is heterogeneous, re-entry might occur.
It is fundamental to understand how re-entry occurs, as this mechanism is responsible for the majority of arrhythmias requiring treatment. The mechanism is somewhat intricate.
Re-entry means that the depolarizing wave front moves around itself in a circle. It is simply an electrical circle loop. This circular movement of the depolarizing wave is referred to as circus movement.
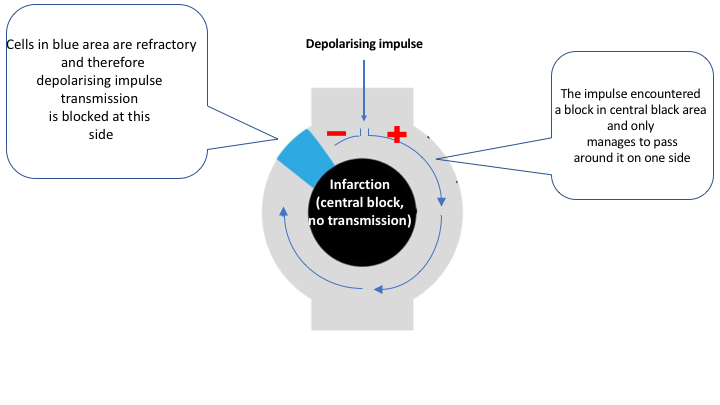
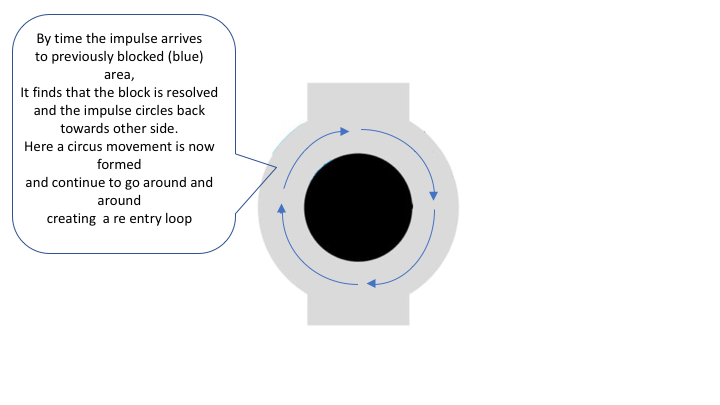
Re-entry occurs if the depolarizing impulse encounters a blocked area (“Central blocking”) that can only be passed on one side. The impulse manages to get around the central blocking on one side, circulates around it and travels back. If the previously blocked area has become excitable by the time the impulse arrives
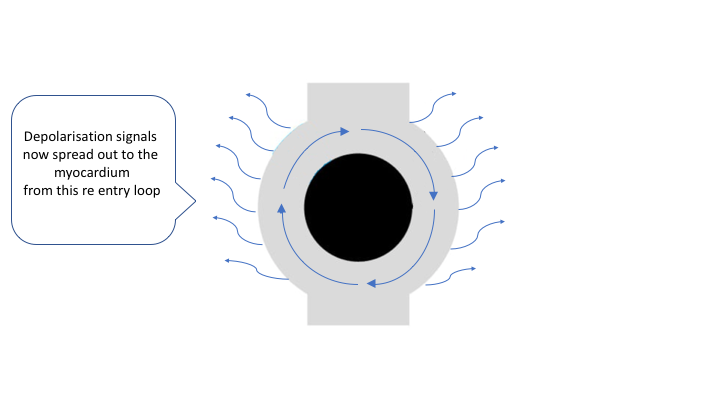
there, it will pass it. The depolarizing wave front will then be able to continue this looping for as long as it encounters excitable tissue. This circus movement is typically very fast and it emits depolarizing impulses
to the surrounding myocardium. Hence, the re-entry circuit generates impulses that activate the myocardium at very high rate.
The prerequisites for re-entry explanation are:
- There must exist a path of electrically connected myocardium. This path should form a circuit, around which the impulse can loop. Any cardiac cell that can carry out an action potential may be part of the circuit. The circuit may vary from a few millimetres to a decimetre in diameter.
- It is crucial that the cells included in the circuit have varying ability to conduct the impulse. This variation is due to differences in refractoriness, conductivity and/or excitability, and it will lead to blocking of the arriving impulse.
- The circuit must surround a core of tissue that cannot be depolarized (central blocking). This core can consist of necrotic myocardium, scar tissue or even valves (the valves have a fibrous ring).
Re-entry is subdivided into functional and anatomical. Knowledge of this distinction is not crucial for clinical practice.
Anatomical re-entry
The explanations outlined above actually apply to anatomical re-entry. In this type of re-entry, the central blocking consists of distinct anatomical structures. For example, atrial flutter (which is a re-entry tachyarrhythmia) arises when the impulse starts to circle around the tricuspid valve. In that scenario the valve is the central blocking (valvular tissue cannot be depolarized) and the circuit is composed of the myocardial fibres surrounding the valve.
Anatomical re-entry is fixed, which means that the location of the re-entry and the speed by which it circulates is constant. It is also a stable re-entry; episodes of atrial flutter may persist for hours or even days.
AVNRT (atrioventricular node re-entrant tachycardia), AVRT (atrioventricular re-entrant tachycardia), most cases of ventricular tachycardia (particularly those originating in the His-Purkinje network, as well as post-infarction cases) are also due to anatomical re-entry.
Functional re-entry
Functional re-re-entry is somewhat more difficult to grasp, because the central blocking and the circuit around is more difficult to define anatomically.
The central blocking and the circuit arise due to electrophysiological heterogeneity (variation) in the myocardium.
Such heterogeneity includes varying refractoriness, conductivity and/or excitability. An impulse traveling through an area with such heterogeneity might encounter a functional block, circulate around it and during its first lap the wave front will emit impulses both outwards and inwards (towards the core of the circuit). The core becomes bombarded with impulses and thus becomes refractory.
Functional re-entry circuits are small, unstable and may engender additional re-entry circuits (this is explained in the article on atrial fibrillation). Functional re-entry is fundamental for development of atrial and ventricular fibrillation.
Clinical significance
The re-entry circuit will die out if the wave front encounters tissue that cannot be excited (depolarized). The wave front must continuously encounter excitable tissue in order to continue its movement. If it encounters non-excitable tissue it will be terminated. The purpose of delivering an electrical shock through the heart (for example during ventricular tachycardia) is to depolarize all excitable cells in the heart, including those involved in the re-entry, whereby the re-entry is terminated (the wave front will encounter refractory cells).
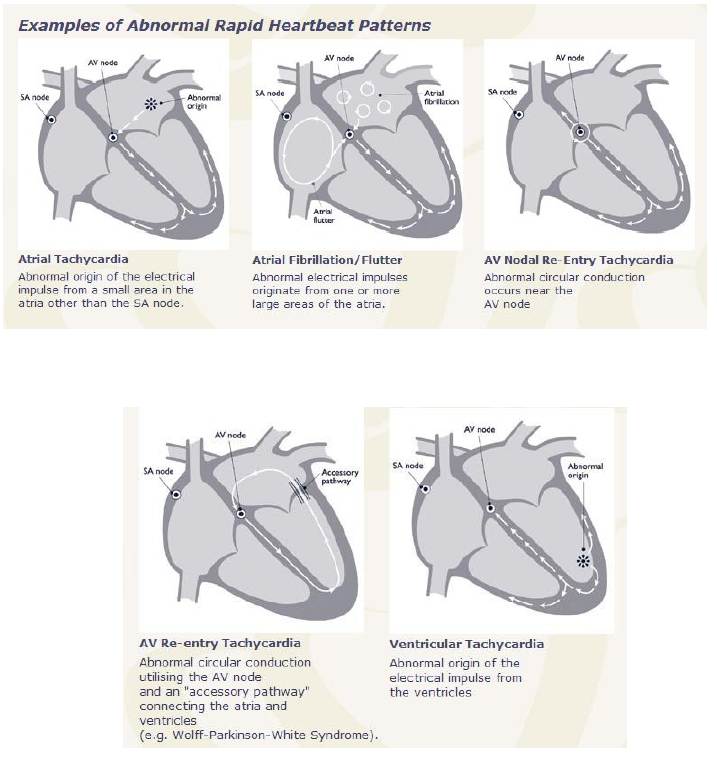
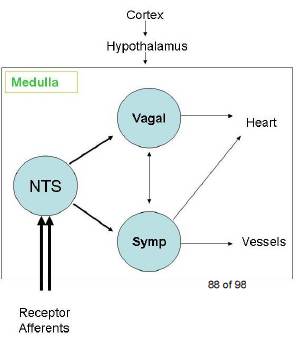
There are three types of tachycardias:
- Atrial or Supraventricular tachycardia (SVT)
- Sinus tachycardia
- Ventricular tachycardia
SVT
Supraventricular tachycardia (SVT) is a generic term that refers to any rapid heart rhythm originating above the ventricular tissue. Although technically an SVT can be due to any supraventricular cause, the term is often used by clinicians to refer to one specific cause of SVT, namely Paroxysmal supraventricular tachycardia (PSVT) which is due to AV nodal reentrant tachycardia.
- AV nodal reentrant tachycardia (AVNRT) is also sometimes referred to as junctional reciprocating tachycardia (JRT) since the atrioventricular junction (AV junction) includes the AV node. It involves a reentry circuit forming just next to or within the AV node itself. The circuit most often involves two tiny pathways one faster than the other, within the AV node. Because the AV node is immediately between the atria and the ventricle, the re-entry circuit often stimulates both, meaning that a retrograde conducted P-wave is buried within or occurs just after the regular, narrow QRS complexes.
Atrioventricular reciprocating tachycardia (AVRT), also known as circus movement tachycardia (CMT), also results from a reentry circuit, although one physically much larger than AVNRT. One portion of the circuit is usually the AV node, and the other, an abnormal accessory pathway from the atria to the ventricle.
Wolff-Parkinson-White syndrome is a relatively common abnormality with an accessory pathway, the Bundle of Kent crossing the AV valvular ring.
In orthodromic AVRT, atrial impulses are conducted down through the AV node and re-enter the atrium via the accessory pathway in a retrograde direction. A distinguishing characteristic of orthodromic AVRT can, therefore, be a P-wave that follows each of its regular, narrow QRS complexes, due to retrograde conduction.
In antidromic AVRT, atrial impulses are conducted down through the accessory pathway and re-enter the atrium retrograde via the AV node. Because the accessory pathway initiates conduction in the ventricles outside of the bundle of His, the QRS complex in antidromic AVRT is often wider than usual, with a delta wave.
Source: http://en.wikipedia.org/wiki/Supraventricular_tachycardia
Symptoms of SVT
Supraventricular tachycardia may come and go suddenly, with stretches of normal heart rates in between. Symptoms may last anywhere from a few minutes to a few days, and some people have no symptoms at all.
Supraventricular tachycardia becomes a problem when it occurs frequently and is ongoing, particularly if there is co-existing heart damage or other medical problems.
Signs and symptoms of supraventricular tachycardia may include:
- A fluttering in the chest
- Rapid heartbeat (palpitations)
- Shortness of breath
- Lightheadedness or dizziness (hemodynamically unstable tachycardia)
- Sweating
- A pounding sensation in the neck
- Fainting (syncope) or near fainting
In infants and very young children, signs and symptoms may be difficult to identify. Sweating, poor feeding, pale skin and infants with a pulse rate greater than 200 beats per minute may have supraventricular tachycardia.
Source: https://www.mayoclinic.org/diseases-conditions/supraventricular-tachycardia/symptoms-causes/syc-20355243
Management of SVT
Vagal manoeuvers
The first-line management for hemodynamically stable patients is vagal maneuvers, such as Valsalva manoeuver. This tends to slow conduction in the AV node and can potentially interrupt the reentrant circuit. Valsalva maneuver, if performed properly by the patient, can frequently terminate the arrhythmia.
Carotid massage is another vagal maneuver that can slow AV nodal conduction. This is performed by massaging the carotid sinus at the bifurcation of the common carotid artery for several seconds on the non-dominant cerebral hemisphere side. Due to the risk of stroke from emboli, auscultate for bruits before attempting this maneuver. Do not perform carotid massage on both sides simultaneously. This maneuver is usually reserved for young patients.
Acute Pharmacologic Management
When SVT is not terminated by vagal maneuvers, short-term management involves intravenous adenosine or calcium channel blockers (performed by a cardiologist). Adenosine is a short-acting drug (~ 10 sec) that blocks AV node conduction; it terminates ~90% of tachycardias due to AVNRT or AVRT.
Other alternatives for the acute treatment of SVT include calcium channel blockers, such as verapamil and diltiazem, as well as beta-blockers, such as metoprolol or esmolol. Verapamil is a calcium channel blocker that also has AV blocking properties. It has a longer half-life than adenosine and may help to maintain sinus rhythm following the termination of SVT.
Long-Term Pharmacologic Management
This largely depends on the type of tachyarrhythmia that is occurring and the frequency and duration of episodes, as well as the symptoms and the risks associated with the arrhythmia (eg, heart failure, sudden death).
Patients with paroxysmal SVT may initially be treated with calcium channel blockers, digoxin, and/or beta-blockers. Class IA, IC, or III antiarrhythmic agents may be used but less frequently because of the success of radiofrequency catheter ablation.
Radiofrequency Catheter Ablation
Catheter ablation is considered the first-line treatment of many recurrent symptomatic SVTs. It is generally performed using conscious sedation in an outpatient setting or with an overnight hospital stay for observation.
Catheter ablation involves focally ablating the component of the arrhythmic mechanism. For example, in AVNRT, the slow pathway is ablated, which prevents the reentry cycle. The accessory pathway is targeted in patients with AVRT. Focal atrial tachycardia, atrial flutter, and, in some cases, atrial fibrillation can also be cured with ablation.
Source: https://emedicine.medscape.com/article/156670-medication
Sinus tachycardia
Sinus tachycardia is a normal increase in the heart rate.
How it happens
The sinoatrial (SA) node --- the heart's natural pacemaker - sends out electrical signals faster than usual. The heart rate is fast, but the heart beats properly.
Causes of sinus tachycardia
A rapid heartbeat may be your body's response to common conditions such as:
- Fever
- Anxiety
- Some medicinal and street drugs
- Severe emotional distress
- Fright
- Strenuous exercise
Other causes but less commonly, it may indicate:
- Anemia (low blood count)
- Increased thyroid activity
- Heart muscle damage from heart attack or heart failure
- Hemorrhage (severe bleeding)
Symptoms
The heart beats faster than usual.
Treatments
Consider and treat the cause of sinus tachycardia rather than the condition itself. If the rapid heartbeat is a symptom of a more serious or longer-term problem, simply slowing the heart rate could cause more harm and leave the underlying condition untreated.
Ventricular tachycardia
is a fast heart rate that starts in the ventricles. It can be a life-threatening heart rhythm and requires rapid diagnosis and treatment.
Causes
Electrical signals in the ventricles fire abnormally, which interferes with electrical signals coming from the sinoatrial (SA) node --- the heart's natural pacemaker. The rapid heartbeat does not allow enough time for the heart to fill before it contracts so blood does not get pumped throughout the body.
It is usually associated with disorders of that heart which interfere with the normal conduction system. These disorders may include:
- Ischaemia to areas of the heart due to lack of coronary artery blood flow
- Cardiomyopathy
- Medications
- Illicit drugs such as cocaine
- Sarcoidosis (an inflammatory disease affecting skin or other body tissues)
Symptoms
- Dizziness
- Palpitations
- Shortness of breath
- Some people might have nausea
- Lightheadedness
- Unconsciousness
- Cardiac arrest
Consequences of Ventricular Tachycardia
This type of arrhythmia may be either well-tolerated or may be life-threatening. The seriousness depends largely on whether another cardiac dysfunction is present, and on the rate of VT.
Treatment
The type and length of treatment depend on the cause.
Treatment may include:
- Immediate electrical therapy (cardioversion / defibrillation)
- Medication (prescribed for home use and/or administered by healthcare professionals)
- Radiofrequency ablation
- Surgery
Source:http://www.heart.org/HEARTORG/Conditions/Arrhythmia/AboutArrhythmia/Tachycardia-Fast-Heart-Rate_UCM_302018_Article.jsp#.W1-IvS2B2OE
Reentry Mechanism
This mechanism may account for most tachyarrhythmias found in patients.
For the normal impulse transmission in the heart, the depolarizing wave needs to spread rapidly and uniformly without interruption through the myocardium. This requires that all the myocardial cells ahead of the impulse wave are excitable and offer the equal capacity to transmit the impulse. In case that the impulse encounters myocardial cells that are not excitable (infarction) or areas where the conductivity is dissimilar, re-entry might occur.
Reentry mechanism

|
Download the video file [0.0 MB] |
Bradycardia
Bradycardia: Heart rate is < 60 bpm and is symptomatic.
Causes of sinus bradycardia include the following:
- The most common cause is the sick sinus syndrome.
- The most common medications responsible include therapeutic and supratherapeutic doses of digitalis glycosides, beta-blockers, and calcium channel blocking agents.
- Other cardiac drugs less commonly implicated include class I antiarrhythmic agents and amiodarone.
- Other drugs and toxins have been reported to cause bradycardia, including lithium, paclitaxel(chemotherapeutic), reserpine, toluene, clonidine, dimethyl sulfoxide(DMSO), topical ophthalmic acetylcholine and opioids e.g. fentanyl.
- Hypothermia, hypoglycemia, hypoxia and electrolyte imbalance.
- The sinus node may be affected as a result of diphtheria, rheumatic fever, or viral myocarditis.
Symptoms:
Sinus bradycardia is most often asymptomatic. However, symptoms may include the following
- Syncope
- Dizziness
- Lightheadedness
- Chest pain
- Shortness of breath
- Exercise intolerance
Assessment (ABCDE) and management: Please refer to bradycardia algorithm
- Maintain a patent airway.
- Assist breathing as needed.
- Administer oxygen if oxygen saturation is less than 94% or the patient is short of breath.
- Monitor blood pressure and heart rate.
- Obtain a 12-lead ECG.
- Establish IV access.
- Complete a focused history and physical examination.
- Search and treat possible contributing factors.
- Signs and symptoms of poor perfusion
If perfusion is poor:
- Consider atropine 0.5 mg IV. This may be repeated every 3 to 5 minutes up to 3mg or 6 doses.
- Prepare for transcutaneous pacing (TCP). Do not delay pacing, if atropine is not effective.
- Consider adrenaline or dopamine while waiting for the pacer or if pacing is ineffective.
- Adrenaline 2 to 10 µg/min
- Dopamine 2 to 20 µg/kg per minute
- Seek expert consultation
Valsalva manoeuvre
against a closed
glottis, changes
occur in intrathoracic
pressure
that dramatically
affects venous
return, cardiac
output, arterial
pressure, and
heart rate. This
forced expiratory
effort is called a Valsalva manoeuvre.
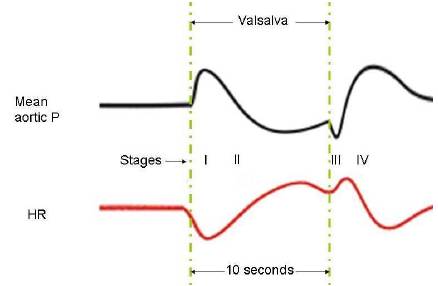
There are four recognisable stages (Phases) in Valsalva:
Initially, during a Valsalva, the intra-thoracic pressure becomes very positive
which compresses the heart and thoracic blood vessels. The venous compression and the accompanying large increase in right atrial pressure impede venous return into the thorax which in turn reduces cardiac filling and preload. Reduced cardiac filling and preload lead to a fall in cardiac output as explained by the Frank-Starling mechanism.
At the same time, compression of the thoracic aorta transiently increases aortic pressure (Stage I); however, aortic pressure begins to fall (Stage II) after a few seconds because cardiac output falls.
Changes in heart rate are almost reciprocal to the changes in the mean aortic pressure due to the operation of the baroreceptor reflex.
During stage I, heart rate decreases because the aortic pressure is elevated; during stage II, as the aortic pressure falls, the heart rate increases. After 10 seconds when the person starts to breathe normally again, aortic pressure briefly decreases as the external compression on the aorta is removed, and heart rate briefly increases reflexively (Stage III). This is followed by an increase in aortic pressure (and a reflex decrease in heart rate) as the cardiac output suddenly increases in response to a rapid increase in the cardiac filling (Stage IV).
Aortic pressure also rises above normal because of the persisting baroreceptor, sympathetic-mediated increase in systemic vascular resistance that occurred during the Valsalva. That leads to increased vagal stimulation (baroreceptors reflex) that may terminate an AVNRT.
Procedure:
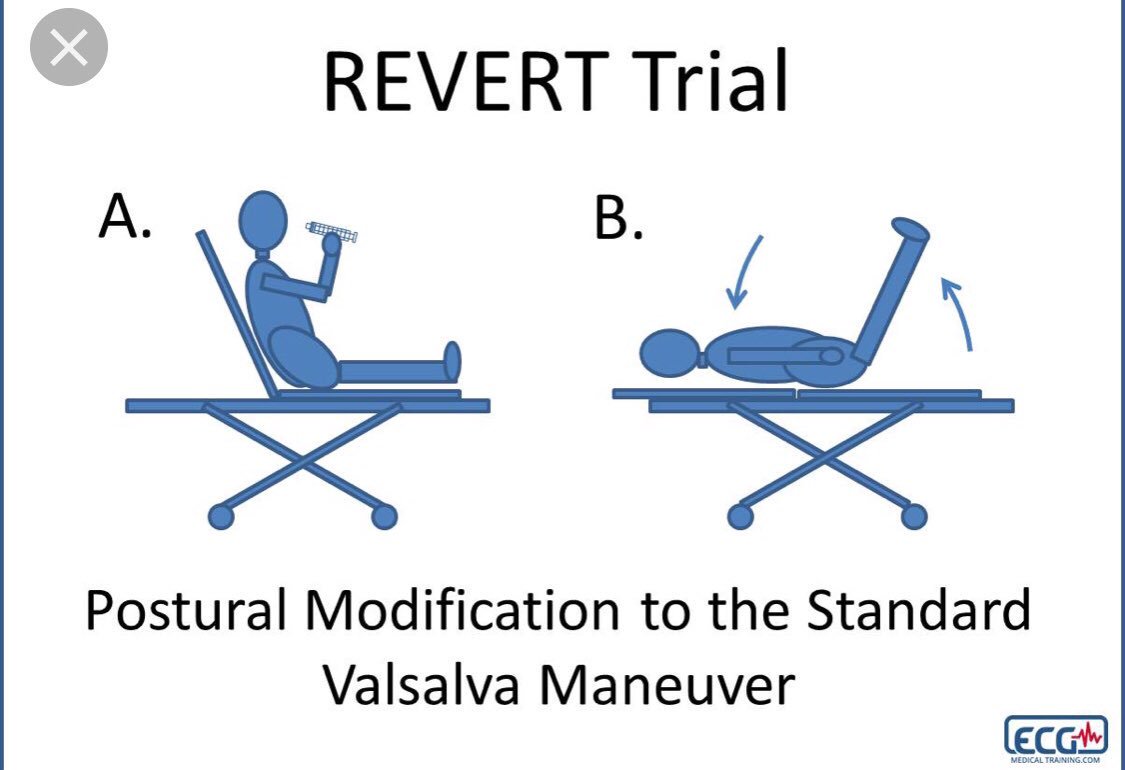
The modified Valsalva manoeuvre strain is standardised to a pressure of about 40 mm Hg sustained for 15 s by forced expiration using a 20-30ml syringe .
Participants, positioned semi-recumbent (at 45°) on a trolley, are directed to perform the strain.
The modified Valsalva manoeuvre is termed “lying down with legs lift Valsalva” performed following the strain in the semi-recumbent position as above but immediately at the end of the strain, the patient is laid flat and the legs raised by to 45° for 15 s. Participants were then returned to the semi-recumbent position for a further 45 s before re-assessment of cardiac rhythm by 3-lead ECG.
Article:

|
Download the video file [0.0 MB] |
Baroreceptors
The carotid sinus baroreceptors (Stretch receptors) are innervated by the sinus nerve of Hering, which is a branch of the glossopharyngeal nerve (IX cranial nerve).
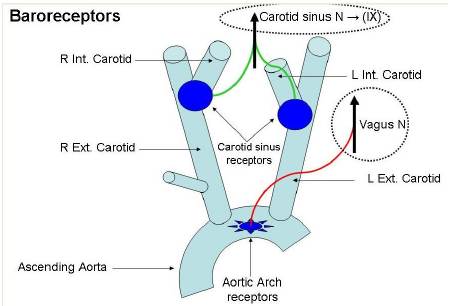
The glossopharyngeal nerve synapses in the nucleus tractus solitarius (NTS) located in the medulla of the brainstem.
The aortic arch baroreceptors are innervated by the aortic nerve, which joins the vagus nerve (X cranial nerve) travelling to the NTS.
The NTS modulates the activity of sympathetic and parasympathetic (vagal) neurons in the medulla, which in turn regulate the autonomic control of the heart and blood vessels.